Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling
- PMID: 20404150
- PMCID: PMC2889580
- DOI: 10.1073/pnas.1000157107
Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling
Abstract
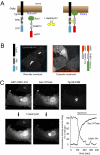
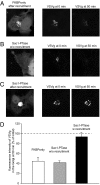
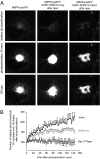
Phosphoinositides are essential lipid regulators of trafficking and signaling pathways of all eukaryotic cells. Phosphatidylinositol 4-phosphate (PtdIns4P) is an intermediate in the synthesis of several important phosphoinositide species but also serves as a regulatory molecule in its own right. Phosphatidylinositol 4-kinases are most abundant in the Golgi but are also found in the plasma membrane and in endocytic compartments. To investigate the role of Golgi PtdIns4P in orchestrating trafficking events, we used a unique drug-inducible molecular approach to rapidly deplete PtdIns4P from Golgi membranes by a recruitable Sac1 phosphatase enzyme. The utility of the system was shown by the rapid loss of Golgi localization of PH domains known to bind PtdIns4P after Sac1 recruitment to the Golgi. Acute PtdIns4P depletion prevented the exit of cargo from the Golgi destined to both the plasma membrane and the late endosomes and led to the loss of some but not all clathrin adaptors from the Golgi membrane. Rapid PtdIns4P depletion in the Golgi also impaired but did not eliminate the replenishment of the plasma membrane PtdIns(4,5)P(2) during phospholipase C activation revealing a hitherto unrecognized contribution of Golgi PtdIns4P to this process. This unique approach will allow further studies on the role of phosphoinositides in endocytic compartments that have evaded detection using the conventional long-term manipulations of inositide kinase and phosphatase activities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
NPC1 regulates the distribution of phosphatidylinositol 4-kinases at Golgi and lysosomal membranes.EMBO J. 2021 Jul 1;40(13):e105990. doi: 10.15252/embj.2020105990. Epub 2021 May 21. EMBO J. 2021. PMID: 34019311 Free PMC article.
-
Local control of phosphatidylinositol 4-phosphate signaling in the Golgi apparatus by Vps74 and Sac1 phosphoinositide phosphatase.Mol Biol Cell. 2012 Jul;23(13):2527-36. doi: 10.1091/mbc.E12-01-0077. Epub 2012 May 2. Mol Biol Cell. 2012. PMID: 22553352 Free PMC article.
-
Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2).Biochem J. 2009 Jul 29;422(1):23-35. doi: 10.1042/BJ20090428. Biochem J. 2009. PMID: 19508231 Free PMC article.
-
The phosphatidylinositol 4-kinases: don't call it a comeback.Subcell Biochem. 2012;58:1-24. doi: 10.1007/978-94-007-3012-0_1. Subcell Biochem. 2012. PMID: 22403072 Review.
-
Phosphatidylinositol 4-phosphate: a key determinant of plasma membrane identity and function in plants.New Phytol. 2022 Aug;235(3):867-874. doi: 10.1111/nph.18258. Epub 2022 Jun 8. New Phytol. 2022. PMID: 35586972 Review.
Cited by
-
PtdIns(4)P regulates retromer-motor interaction to facilitate dynein-cargo dissociation at the trans-Golgi network.Nat Cell Biol. 2013 Apr;15(4):417-29. doi: 10.1038/ncb2710. Epub 2013 Mar 24. Nat Cell Biol. 2013. PMID: 23524952
-
NPC1 regulates the distribution of phosphatidylinositol 4-kinases at Golgi and lysosomal membranes.EMBO J. 2021 Jul 1;40(13):e105990. doi: 10.15252/embj.2020105990. Epub 2021 May 21. EMBO J. 2021. PMID: 34019311 Free PMC article.
-
An update on genetically encoded lipid biosensors.Mol Biol Cell. 2022 May 1;33(5):tp2. doi: 10.1091/mbc.E21-07-0363. Mol Biol Cell. 2022. PMID: 35420888 Free PMC article.
-
A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi.J Cell Biol. 2014 Apr 14;205(1):113-26. doi: 10.1083/jcb.201312072. Epub 2014 Apr 7. J Cell Biol. 2014. PMID: 24711504 Free PMC article.
-
Multiphasic dynamics of phosphatidylinositol 4-phosphate during phagocytosis.Mol Biol Cell. 2017 Jan 1;28(1):128-140. doi: 10.1091/mbc.E16-06-0451. Epub 2016 Nov 9. Mol Biol Cell. 2017. PMID: 28035045 Free PMC article.
References
-
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
-
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001(111):re19. - PubMed
-
- Balla A, Balla T. Phosphatidylinositol 4-kinases: Old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

