H-NOX-mediated nitric oxide sensing modulates symbiotic colonization by Vibrio fischeri
- PMID: 20404170
- PMCID: PMC2889544
- DOI: 10.1073/pnas.1003571107
H-NOX-mediated nitric oxide sensing modulates symbiotic colonization by Vibrio fischeri
Abstract
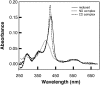
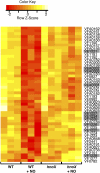
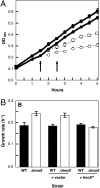
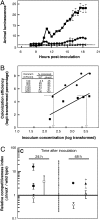
The bioluminescent bacterium Vibrio fischeri initiates a specific, persistent symbiosis in the light organ of the squid Euprymna scolopes. During the early stages of colonization, V. fischeri is exposed to host-derived nitric oxide (NO). Although NO can be both an antimicrobial component of innate immunity and a key signaling molecule in eukaryotes, potential roles in beneficial host-microbe associations have not been described. V. fischeri hnoX encodes a heme NO/oxygen-binding (H-NOX) protein, a member of a family of bacterial NO- and/or O(2)-binding proteins of unknown function. We hypothesized that H-NOX acts as a NO sensor that is involved in regulating symbiosis-related genes early in colonization. Whole-genome expression studies identified 20 genes that were repressed in an NO- and H-NOX-dependent fashion. Ten of these, including hemin-utilization genes, have a promoter with a putative ferric-uptake regulator (Fur) binding site. As predicted, in the presence of NO, wild-type V. fischeri grew more slowly on hemin than a hnoX deletion mutant. Host-colonization studies showed that the hnoX mutant was also 10-fold more efficient in initially colonizing the squid host than the wild type; similarly, in mixed inoculations, it outcompeted the wild-type strain by an average of 16-fold after 24 h. However, the presence of excess hemin or iron reversed this dominance. The advantage of the mutant in colonizing the iron-limited light-organ tissues is caused, at least in part, by its greater ability to acquire host-derived hemin. Our data suggest that V. fischeri normally senses a host-generated NO signal through H-NOX(Vf) and modulates the expression of its iron uptake capacity during the early stages of the light-organ symbiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L, McFall-Ngai MJ. NO means ‘yes’ in the squid-vibrio symbiosis: Nitric oxide (NO) during the initial stages of a beneficial association. Cell Microbiol. 2004;6:1139–1151. - PubMed
-
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. - PubMed
-
- Spiro S. Regulators of bacterial responses to nitric oxide. FEMS Microbiol Rev. 2007;31:193–211. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

