Induction of reactive oxygen species-mediated autophagy by a novel microtubule-modulating agent
- PMID: 20404319
- PMCID: PMC2881797
- DOI: 10.1074/jbc.M109.091694
Induction of reactive oxygen species-mediated autophagy by a novel microtubule-modulating agent
Abstract
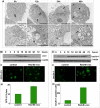
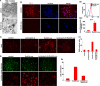
Autophagy is being increasingly implicated in both cell survival and death. However, the intricate relationships between drug-induced autophagy and apoptosis remain elusive. Here we demonstrate that a tubulin-binding noscapine analog, (R)-9-bromo-5-((S)-4,5-dimethoxy-1,3-dihydroisobenzofuran-1-yl)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]-di-oxolo[4,5-g]isoquinoline (Red-Br-nos), exerts a novel autophagic response followed by apoptotic cell death in human prostate cancer PC-3 cells. Red-Br-nos-induced autophagy was an early event detectable within 12 h that displayed a wide array of characteristic features including double membranous vacuoles with entrapped organelles, acidic vesicular organelles, and increased expression of LC3-II and beclin-1. Red-Br-nos-triggered release of reactive oxygen species (ROS) and attenuation of ROS by tiron, a ROS scavenger, reduced the sub-G(1) population suggesting ROS-dependent apoptosis. Abrogation of ROS also reduced autophagy indicating that ROS triggers autophagy. Pharmacological and genetic approaches to inhibit autophagy uncovered the protective role of Red-Br-nos-induced autophagy in PC-3 cells. Direct effects of the drug on mitochondria viz. disruption of normal cristae architecture and dissipation of mitochondrial transmembrane potential revealed a functional link between ROS generation, autophagy, and apoptosis induction. This is the first report to demonstrate the protective role of ROS-mediated autophagy and induction of caspase-independent ROS-dependent apoptosis in PC-3 cells by Red-Br-nos, a member of the noscapinoid family of microtubule-modulating anticancer agents.
Figures




Similar articles
-
Induction of robust de novo centrosome amplification, high-grade spindle multipolarity and metaphase catastrophe: a novel chemotherapeutic approach.Cell Death Dis. 2012 Jul 12;3(7):e346. doi: 10.1038/cddis.2012.82. Cell Death Dis. 2012. PMID: 22785532 Free PMC article.
-
9-PAN promotes tubulin- and ROS-mediated cell death in human triple-negative breast cancer cells.J Pharm Pharmacol. 2020 Nov;72(11):1585-1594. doi: 10.1111/jphp.13349. Epub 2020 Aug 16. J Pharm Pharmacol. 2020. PMID: 32959391
-
Design, synthesis and biological evaluation of di-substituted noscapine analogs as potent and microtubule-targeted anticancer agents.Bioorg Med Chem Lett. 2015;25(10):2133-40. doi: 10.1016/j.bmcl.2015.03.076. Epub 2015 Mar 31. Bioorg Med Chem Lett. 2015. PMID: 25891106 Free PMC article.
-
Insight into the Tubulin-Targeted Anticancer Potential of Noscapine and its Structural Analogs.Anticancer Agents Med Chem. 2023;23(6):624-641. doi: 10.2174/1871520622666220804115551. Anticancer Agents Med Chem. 2023. PMID: 35927808 Review.
-
Mitochondrial ROS generation for regulation of autophagic pathways in cancer.Biochem Biophys Res Commun. 2011 Oct 14;414(1):5-8. doi: 10.1016/j.bbrc.2011.09.046. Epub 2011 Sep 17. Biochem Biophys Res Commun. 2011. PMID: 21951851 Review.
Cited by
-
Centrosome-declustering drugs mediate a two-pronged attack on interphase and mitosis in supercentrosomal cancer cells.Cell Death Dis. 2014 Nov 20;5(11):e1538. doi: 10.1038/cddis.2014.505. Cell Death Dis. 2014. PMID: 25412316 Free PMC article.
-
Potent Ruthenium-Ferrocene Bimetallic Antitumor Antiangiogenic Agent That Circumvents Platinum Resistance: From Synthesis and Mechanistic Studies to In Vivo Evaluation in Zebrafish.J Med Chem. 2022 Dec 22;65(24):16353-16371. doi: 10.1021/acs.jmedchem.2c01174. Epub 2022 Dec 2. J Med Chem. 2022. PMID: 36459415 Free PMC article.
-
Neutrophil autophagy and NETosis in COVID-19: perspectives.Autophagy. 2023 Mar;19(3):758-767. doi: 10.1080/15548627.2022.2099206. Epub 2022 Aug 11. Autophagy. 2023. PMID: 35951555 Free PMC article. Review.
-
Comparison of human lung cancer cell radiosensitivity after irradiations with therapeutic protons and carbon ions.Exp Biol Med (Maywood). 2017 May;242(10):1015-1024. doi: 10.1177/1535370216669611. Epub 2016 Sep 15. Exp Biol Med (Maywood). 2017. PMID: 27633574 Free PMC article.
-
The Noscapine Chronicle: A Pharmaco-Historic Biography of the Opiate Alkaloid Family and its Clinical Applications.Med Res Rev. 2015 Sep;35(5):1072-96. doi: 10.1002/med.21357. Epub 2015 Jul 14. Med Res Rev. 2015. PMID: 26179481 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

