Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons
- PMID: 20404321
- PMCID: PMC2881816
- DOI: 10.1074/jbc.M110.103028
Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons
Erratum in
-
Correction: Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons.J Biol Chem. 2020 Jul 3;295(27):9265. doi: 10.1074/jbc.AAC120.014663. J Biol Chem. 2020. PMID: 32620693 Free PMC article. No abstract available.
Abstract
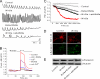
A role for Wnt signal transduction in the development and maintenance of brain structures is widely acknowledged. Recent studies have suggested that Wnt signaling may be essential for synaptic plasticity and neurotransmission. However, the direct effect of a Wnt protein on synaptic transmission had not been demonstrated. Here we show that nanomolar concentrations of purified Wnt3a protein rapidly increase the frequency of miniature excitatory synaptic currents in embryonic rat hippocampal neurons through a mechanism involving a fast influx of calcium from the extracellular space, induction of post-translational modifications on the machinery involved in vesicle exocytosis in the presynaptic terminal leading to spontaneous Ca(2+) transients. Our results identify the Wnt3a protein and a member of its complex receptor at the membrane, the low density lipoprotein receptor-related protein 6 (LRP6) coreceptor, as key molecules in neurotransmission modulation and suggest cross-talk between canonical and Wnt/Ca(2+) signaling in central neurons.
Figures
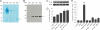

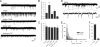


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

