Amelogenin-collagen interactions regulate calcium phosphate mineralization in vitro
- PMID: 20404336
- PMCID: PMC2885206
- DOI: 10.1074/jbc.M109.079939
Amelogenin-collagen interactions regulate calcium phosphate mineralization in vitro
Abstract
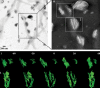
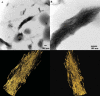
Collagen and amelogenin are two major extracellular organic matrix proteins of dentin and enamel, the mineralized tissues comprising a tooth crown. They both are present at the dentin-enamel boundary (DEB), a remarkably robust interface holding dentin and enamel together. It is believed that interactions of dentin and enamel protein assemblies regulate growth and structural organization of mineral crystals at the DEB, leading to a continuum at the molecular level between dentin and enamel organic and mineral phases. To gain insight into the mechanisms of the DEB formation and structural basis of its mechanical resiliency we have studied the interactions between collagen fibrils, amelogenin assemblies, and forming mineral in vitro, using electron microscopy. Our data indicate that collagen fibrils guide assembly of amelogenin into elongated chain or filament-like structures oriented along the long axes of the fibrils. We also show that the interactions between collagen fibrils and amelogenin-calcium phosphate mineral complexes lead to oriented deposition of elongated amorphous mineral particles along the fibril axes, triggering mineralization of the bulk of collagen fibril. The resulting structure was similar to the mineralized collagen fibrils found at the DEB, with arrays of smaller well organized crystals inside the collagen fibrils and bundles of larger crystals on the outside of the fibrils. These data suggest that interactions between collagen and amelogenin might play an important role in the formation of the DEB providing structural continuity between dentin and enamel.
Figures






References
-
- Nanci A. (2007) Ten Cate's Oral Histology: Development, Structure, and Function, Mosby, St. Louis, MO
-
- Weiner S., Wagner H. D. (1998) Annu. Rev. Materials Sci. 28, 271–298
-
- Landis W. J., Hodgens K. J., Arena J., Song M. J., McEwen B. F. (1996) Microsc. Res. Tech. 33, 192–202 - PubMed
-
- Weiner S., Traub W. (1992) FASEB J. 6, 879–885 - PubMed
-
- Brodsky B., Ramshaw J. A. M. (1997) Matrix Biol. 15, 545–554 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

