Versican facilitates chondrocyte differentiation and regulates joint morphogenesis
- PMID: 20404343
- PMCID: PMC2898371
- DOI: 10.1074/jbc.M109.096479
Versican facilitates chondrocyte differentiation and regulates joint morphogenesis
Abstract
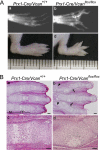
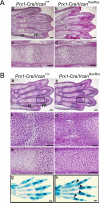
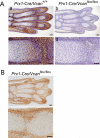
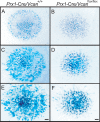
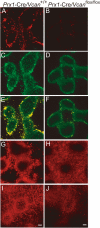
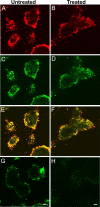
Versican/PG-M is a large chondroitin sulfate proteoglycan in the extracellular matrix, which is transiently expressed in mesenchymal condensation areas during tissue morphogenesis. Here, we generated versican conditional knock-out mice Prx1-Cre/Vcan(flox/flox), in which Vcan is pruned out by site-specific Cre recombinase driven by the Prx1 promoter. Although Prx1-Cre/Vcan(flox/flox) mice are viable and fertile, they develop distorted digits. Histological analysis of newborn mice reveals hypertrophic chondrocytic nodules in cartilage, tilting of the joint, and a slight delay of chondrocyte differentiation in digits. By immunostaining, whereas the joint interzone of Prx1-Cre/Vcan(+/+) shows an accumulation of TGF-beta, concomitant with versican, that of Prx1-Cre/Vcan(flox/flox) without versican expression exhibits a decreased incorporation of TGF-beta. In a micromass culture system of mesenchymal cells from limb bud, whereas TGF-beta and versican are co-localized in the perinodular regions of developing cartilage in Prx1-Cre/Vcan(+/+), TGF-beta is widely distributed in Prx1-Cre/Vcan(flox/flox). These results suggest that versican facilitates chondrogenesis and joint morphogenesis, by localizing TGF-beta in the extracellular matrix and regulating its signaling.
Figures


References
-
- Kimata K., Oike Y., Tani K., Shinomura T., Yamagata M., Uritani M., Suzuki S. (1986) J. Biol. Chem. 261, 13517–13525 - PubMed
-
- Matsumoto K., Shionyu M., Go M., Shimizu K., Shinomura T., Kimata K., Watanabe H. (2003) J. Biol. Chem. 278, 41205–41212 - PubMed
-
- Isogai Z., Aspberg A., Keene D. R., Ono R. N., Reinhardt D. P., Sakai L. Y. (2002) J. Biol. Chem. 277, 4565–4572 - PubMed
-
- Olin A. I., Mörgelin M., Sasaki T., Timpl R., Heinegård D., Aspberg A. (2001) J. Biol. Chem. 276, 1253–1261 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

