Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial
- PMID: 20404379
- PMCID: PMC2946369
- DOI: 10.7326/0003-4819-152-8-201004200-00005
Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial
Abstract
Background: Randomized data on statins for primary prevention in older persons are limited, and the relative hazard of cardiovascular disease associated with an elevated cholesterol level weakens with advancing age.
Objective: To assess the efficacy and safety of rosuvastatin in persons 70 years or older.
Design: Secondary analysis of JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), a randomized, double-blind, placebo-controlled trial.
Setting: 1315 sites in 26 countries randomly assigned participants in JUPITER.
Participants: Among the 17 802 participants randomly assigned with low-density lipoprotein (LDL) cholesterol levels less than 3.37 mmol/L (<130 mg/dL) and high-sensitivity C-reactive protein levels of 2.0 mg/L or more without cardiovascular disease, 5695 were 70 years or older.
Intervention: Participants were randomly assigned in a 1:1 ratio to receive 20 mg of rosuvastatin daily or placebo.
Measurements: The primary end point was the occurrence of a first cardiovascular event (myocardial infarction, stroke, arterial revascularization, hospitalization for unstable angina, or death from cardiovascular causes).
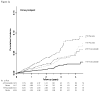
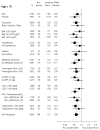
Results: The 32% of trial participants 70 years or older accrued 49% (n = 194) of the 393 confirmed primary end points. The rates of the primary end point in this age group were 1.22 and 1.99 per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio, 0.61 [95% CI, 0.46 to 0.82]; P < 0.001). Corresponding rates of all-cause mortality in this age group were 1.63 and 2.04 (hazard ratio, 0.80 [CI, 0.62 to 1.04]; P = 0.090). Although no significant heterogeneity was found in treatment effects by age, absolute reductions in event rates associated with rosuvastatin were greater in older persons. The relative rate of any serious adverse event among older persons in the rosuvastatin versus placebo group was 1.05 (CI, 0.93 to 1.17).
Limitation: Effect estimates from this exploratory analysis with age cut-point chosen after trial completion should be viewed in the context of the overall trial results.
Conclusion: In apparently healthy older persons without hyperlipidemia but with elevated high-sensitivity C-reactive protein levels, rosuvastatin reduces the incidence of major cardiovascular events.
Primary funding source: AstraZeneca.
Figures



Comment in
-
Summaries for patients. Rosuvastatin to prevent heart problems and stroke in persons 70 years or older.Ann Intern Med. 2010 Apr 20;152(8):I34. doi: 10.7326/0003-4819-152-8-201004200-00002. Ann Intern Med. 2010. PMID: 20404365 No abstract available.
-
Statins for primary prevention in older adults: who is high risk, who is old, and what denotes primary prevention?Ann Intern Med. 2010 Apr 20;152(8):528-30, W183. doi: 10.7326/0003-4819-152-8-201004200-00011. Ann Intern Med. 2010. PMID: 20404384 No abstract available.
References
-
- Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA. 2004;291:1864–1870. - PubMed
-
- Setoguchi S, Glynn RJ, Avorn J, Levin R, Winkelmayer WC. Ten-year trends of cardiovascular drug use after myocardial infarction among community-dwelling persons > or =65 years of age. Am J Cardiol. 2007;100:1061–7. - PubMed
-
- Abramson J, Wright JM. Are lipid-lowering guidelines evidence based? Lancet. 2007;369:168–169. - PubMed
-
- Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. Erratum in: Lancet. 2008; 372:292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
