A growing family: adding mutated Erbb4 as a novel cancer target
- PMID: 20404484
- PMCID: PMC3384517
- DOI: 10.4161/cc.9.8.11239
A growing family: adding mutated Erbb4 as a novel cancer target
Abstract
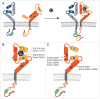
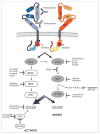
As the upward spiral of novel cancer gene discoveries and novel molecular compounds continues to accelerate, a repetitive theme in molecular drug development remains the lack of activity of initially promising agents when given to patients in clinical trials. It is however invigorating that a few targeted agents directed against a select group of a few 'cancer gene superfamilies' have escaped this all to common fate, and have evolved into novel, clinically meaningful molecular therapy strategies. Targeting dysregulated signaling of the epidermal growth factor family of transmembrane receptors (Erbb family) has encompassed over the last decade an ever increasing role in personalized treatment approaches in an increasing number of human malignancies. Erbbs are receptor tyrosine kinases that are important regulators of several signaling pathways. Two of its family members (Erbb1/EGFR and Erbb2/HER2) have previously been shown to be somatically mutated in large fraction of human cancers. To determine if this family is somatically mutated in melanoma, its sequences were recently analyzed and one of its members, Erbb4, was found to be somatically mutated in 19% of melanoma cases. Functional analysis of seven of its mutations was shown to increase its catalytic and transformation abilities as well as providing essential survival signals. Similar to other Erbb family members, mutant Erbb4 seems to confer 'oncogene addiction' on melanoma cells, making it an attractive therapeutic target. Gaining further understanding into the oncogenic mechanism of Erbb4 may not only help in the development of targeted therapy in melanoma patients but might accelerate the acceptance of a novel taxonomy of cancer which is based on the genomic perturbations in cancer genes and cancer gene families and their response to targeted agents.
Figures

Similar articles
-
ERBB4 mutation analysis: emerging molecular target for melanoma treatment.Methods Mol Biol. 2014;1102:461-80. doi: 10.1007/978-1-62703-727-3_24. Methods Mol Biol. 2014. PMID: 24258993 Free PMC article.
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
-
Pan-erbB inhibition potentiates BRAF inhibitors for melanoma treatment.Melanoma Res. 2014 Jun;24(3):207-18. doi: 10.1097/CMR.0000000000000060. Melanoma Res. 2014. PMID: 24709886 Free PMC article.
-
Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4.Nat Genet. 2009 Oct;41(10):1127-32. doi: 10.1038/ng.438. Epub 2009 Aug 30. Nat Genet. 2009. PMID: 19718025 Free PMC article.
Cited by
-
ERBB4 mutation analysis: emerging molecular target for melanoma treatment.Methods Mol Biol. 2014;1102:461-80. doi: 10.1007/978-1-62703-727-3_24. Methods Mol Biol. 2014. PMID: 24258993 Free PMC article.
-
New therapies in the treatment of melanoma.Expert Opin Investig Drugs. 2012 Nov;21(11):1643-59. doi: 10.1517/13543784.2012.713938. Epub 2012 Aug 9. Expert Opin Investig Drugs. 2012. PMID: 22876817 Free PMC article. Review.
-
Collections of simultaneously altered genes as biomarkers of cancer cell drug response.Cancer Res. 2013 Mar 15;73(6):1699-708. doi: 10.1158/0008-5472.CAN-12-3122. Epub 2013 Jan 21. Cancer Res. 2013. PMID: 23338612 Free PMC article.
-
The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer.Br J Cancer. 2011 Apr 12;104(8):1241-5. doi: 10.1038/bjc.2011.62. Epub 2011 Mar 1. Br J Cancer. 2011. PMID: 21364581 Free PMC article. Review.
-
Pathology and Molecular Biology of Melanoma.Curr Issues Mol Biol. 2023 Jun 30;45(7):5575-5597. doi: 10.3390/cimb45070352. Curr Issues Mol Biol. 2023. PMID: 37504268 Free PMC article. Review.
References
-
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. - PubMed
-
- Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. - PubMed
-
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
