The effects of the histone deacetylase inhibitor romidepsin (FK228) are enhanced by aspirin (ASA) in COX-1 positive ovarian cancer cells through augmentation of p21
- PMID: 20404564
- PMCID: PMC4047717
- DOI: 10.4161/cbt.9.11.11873
The effects of the histone deacetylase inhibitor romidepsin (FK228) are enhanced by aspirin (ASA) in COX-1 positive ovarian cancer cells through augmentation of p21
Abstract
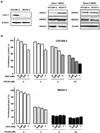
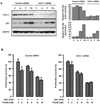
Histone deacetylase (HDAC) inhibitors have shown preclinical efficacy in solid tumors, including ovarian cancers. Our group has published that the HDAC inhibitor, romidepsin (FK228) suppresses ovarian cancer cell growth at nanomolar concentrations in vitro. HDAC inhibitors appear to be even more effective when used in combination with other antitumor agents. However, it remains unclear which antitumor agents are best suited for combination therapy. A recent report suggested that aspirin (acetylsalicylic acid, ASA ) is synergistic with HDAC inhibitors in ovarian cancer cells. ASA is a relatively selective inhibitor of cyclooxygenase-1 (COX-1) and has anti-proliferative effects in ovarian cancer cells. The goal of this study was to investigate the impact of ASA on the activity of the HDAC inhibitor, FK228 in COX-1 positive (OVCAR-3) and COX-1 negative (SKOV-3) human ovarian cancer cell lines. The growth inhibitory effects of FK228 were enhanced by ASA in COX-1 positive ovarian cancer cells. In contrast, ASA had no influence on the results of FK228 treatment in COX-1 negative ovarian cancer cells. Upregulation of the cell cycle control protein p21 was induced robustly by FK228 in both cell lines. In the COX-1 positive cells, p21 expression was augmented by the addition of ASA to FK228 treatment. Furthermore, COX-1 siRNA attenuated the effects of combined ASA and FK228 on the levels of p21 expression and the amount of growth inhibition. The additional increase in p21 by ASA in FK228-treated cells was not observed at the promoter or transcriptional levels. However, a significant delay in p21 protein degradation in the presence of ASA and FK228 in COX-1 positive cells was associated with inhibition of proteasome activity. Our study provides a potential rationale for combining ASA with HDAC inhibitors in a subset of ovarian cancers.
Figures




Similar articles
-
Histone deacetylase inhibitor, Romidepsin (FK228) inhibits endometrial cancer cell growth through augmentation of p53-p21 pathway.Biomed Pharmacother. 2016 Aug;82:161-6. doi: 10.1016/j.biopha.2016.04.053. Epub 2016 May 9. Biomed Pharmacother. 2016. PMID: 27470351
-
Romidepsin (FK228) and its analogs directly inhibit phosphatidylinositol 3-kinase activity and potently induce apoptosis as histone deacetylase/phosphatidylinositol 3-kinase dual inhibitors.Cancer Sci. 2012 Nov;103(11):1994-2001. doi: 10.1111/cas.12002. Epub 2012 Oct 12. Cancer Sci. 2012. PMID: 22924958 Free PMC article.
-
Biochemical, biological and structural properties of romidepsin (FK228) and its analogs as novel HDAC/PI3K dual inhibitors.Cancer Sci. 2015 Feb;106(2):208-15. doi: 10.1111/cas.12585. Epub 2015 Jan 28. Cancer Sci. 2015. PMID: 25492515 Free PMC article.
-
Romidepsin (FK228), A Histone Deacetylase Inhibitor and its Analogues in Cancer Chemotherapy.Curr Med Chem. 2021;28(7):1290-1303. doi: 10.2174/0929867327666200203113926. Curr Med Chem. 2021. PMID: 32013816 Review.
-
Histone deacetylase inhibitors from microorganisms: the Astellas experience.Prog Drug Res. 2008;66:335, 337-59. doi: 10.1007/978-3-7643-8595-8_7. Prog Drug Res. 2008. PMID: 18416310 Review.
Cited by
-
Combined histone deacetylase and cyclooxygenase inhibition achieves enhanced antiangiogenic effects in lung cancer cells.Mol Carcinog. 2013 Mar;52(3):218-28. doi: 10.1002/mc.21846. Epub 2011 Nov 28. Mol Carcinog. 2013. PMID: 22121107 Free PMC article.
-
Combinatorial Antitumor Activity of Oxaliplatin with Epigenetic Modifying Agents, 5-Aza-CdR and FK228, in Human Gastric Cancer Cells.Biomol Ther (Seoul). 2018 Nov 1;26(6):591-598. doi: 10.4062/biomolther.2018.061. Biomol Ther (Seoul). 2018. PMID: 30173503 Free PMC article.
-
Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature.Pharmaceuticals (Basel). 2018 Oct 11;11(4):101. doi: 10.3390/ph11040101. Pharmaceuticals (Basel). 2018. PMID: 30314310 Free PMC article. Review.
-
Epigenetic Regulation: A Link between Inflammation and Carcinogenesis.Cancers (Basel). 2022 Feb 26;14(5):1221. doi: 10.3390/cancers14051221. Cancers (Basel). 2022. PMID: 35267528 Free PMC article. Review.
-
Highly pathogenic porcine reproductive and respiratory syndrome virus induces prostaglandin E2 production through cyclooxygenase 1, which is dependent on the ERK1/2-p-C/EBP-β pathway.J Virol. 2014 Mar;88(5):2810-20. doi: 10.1128/JVI.03205-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352469 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics 2009. CA Cancer J Clin. 2009 - PubMed
-
- Chobanian N, Dietrich CS., 3rd Ovarian cancer. Surg Clin North Am. 2008;88:285–299. vi. - PubMed
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. - PubMed
-
- Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. - PubMed
-
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
