Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness
- PMID: 20404739
- PMCID: PMC3174771
- DOI: 10.1097/QAI.0b013e3181d0db97
Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness
Abstract
Background: As second-line antiretroviral therapy (ART) availability increases in resource-limited settings, questions about the value of laboratory monitoring remain. We assessed the outcomes and cost-effectiveness (CE) of laboratory monitoring to guide switching ART.
Methods: We used a computer model to project life expectancy and costs of different strategies to guide ART switching in patients in Côte d'Ivoire. Strategies included clinical assessment, CD4 count, and HIV RNA testing. Data were from clinical trials and cohort studies from Côte d'Ivoire and the literature. Outcomes were compared using the incremental CE ratio. We conducted multiple sensitivity analyses to assess uncertainty in model parameters.
Results: Compared with first-line ART only, second-line ART increased life expectancy by 24% with clinical monitoring only, 46% with CD4 monitoring, and 61% with HIV RNA monitoring. The incremental CE ratio of switching based on clinical monitoring was $1670 per year of life gained (YLS) compared with first-line ART only; biannual CD4 monitoring was $2120 per YLS. The CE ratio of biannual HIV RNA testing ranged from $2920 ($87/test) to $1990 per YLS ($25/test). If second-line ART costs were reduced, the CE of HIV RNA monitoring improved.
Conclusions: In resource-limited settings, CD4 count and HIV RNA monitoring to guide switching to second-line ART improve survival and, under most conditions, are cost-effective.
Figures
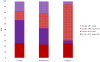
 ) and dark purple (
) and dark purple (
 ), respectively. Time on suppressed and virologically failed 2nd-line ART is shown in lighter red (
), respectively. Time on suppressed and virologically failed 2nd-line ART is shown in lighter red (
 ) and lighter purple (
) and lighter purple (
 ), respectively. Detecting ART failure earlier — as occurs when using HIV RNA monitoring — resulted in a shorter duration on virologically failed 1st-line ART and longer total duration on 2nd-line ART. ART: antiretroviral therapy.
), respectively. Detecting ART failure earlier — as occurs when using HIV RNA monitoring — resulted in a shorter duration on virologically failed 1st-line ART and longer total duration on 2nd-line ART. ART: antiretroviral therapy.



Similar articles
-
Cost-effectiveness and budget impact of immediate antiretroviral therapy initiation for treatment of HIV infection in Côte d'Ivoire: A model-based analysis.PLoS One. 2019 Jun 27;14(6):e0219068. doi: 10.1371/journal.pone.0219068. eCollection 2019. PLoS One. 2019. PMID: 31247009 Free PMC article.
-
Laboratory Monitoring of Antiretroviral Therapy for HIV Infection: Cost-Effectiveness and Budget Impact of Current and Novel Strategies.Clin Infect Dis. 2016 Jun 1;62(11):1454-1462. doi: 10.1093/cid/ciw117. Epub 2016 Mar 1. Clin Infect Dis. 2016. PMID: 26936666 Free PMC article.
-
Cost-effectiveness of HIV treatment in resource-poor settings--the case of Côte d'Ivoire.N Engl J Med. 2006 Sep 14;355(11):1141-53. doi: 10.1056/NEJMsa060247. N Engl J Med. 2006. PMID: 16971720
-
Economic evaluation of ART in resource-limited countries.Curr Opin HIV AIDS. 2010 May;5(3):225-31. doi: 10.1097/COH.0b013e3283384a9d. Curr Opin HIV AIDS. 2010. PMID: 20539078 Free PMC article. Review.
-
Challenges in implementing HIV laboratory monitoring in resource-constrained settings: how to do more with less.Future Microbiol. 2011 Nov;6(11):1251-60. doi: 10.2217/fmb.11.121. Future Microbiol. 2011. PMID: 22082287 Review.
Cited by
-
Regional differences in predictive accuracy of WHO immunologic failure criteria.AIDS. 2012 Mar 27;26(6):768-70. doi: 10.1097/QAD.0b013e32835143e3. AIDS. 2012. PMID: 22269974 Free PMC article.
-
Sustainable HIV treatment in Africa through viral-load-informed differentiated care.Nature. 2015 Dec 3;528(7580):S68-76. doi: 10.1038/nature16046. Nature. 2015. PMID: 26633768 Free PMC article.
-
Could early antiretroviral therapy entail more risks than benefits in sub-Saharan African HIV-infected adults? A model-based analysis.Antivir Ther. 2013;18(1):45-55. doi: 10.3851/IMP2231. Epub 2012 Jul 18. Antivir Ther. 2013. PMID: 22809695 Free PMC article.
-
Initiation of antiretroviral therapy.Indian J Sex Transm Dis AIDS. 2014 Jan;35(1):1-11. doi: 10.4103/0253-7184.132399. Indian J Sex Transm Dis AIDS. 2014. PMID: 24958979 Free PMC article. Review.
-
Predictors of treatment failure on second-line antiretroviral therapy among adults in northwest Ethiopia: a multicentre retrospective follow-up study.BMJ Open. 2016 Dec 8;6(12):e012537. doi: 10.1136/bmjopen-2016-012537. BMJ Open. 2016. PMID: 27932339 Free PMC article.
References
-
- Kuritzkes DR. Preventing and managing antiretroviral drug resistance. AIDS Patient Care STDS. 2004 May;18(5):259–273. - PubMed
-
- Lee KJ, Dunn D, Porter K, et al. Treatment switches after viral rebound in HIV-infected adults starting antiretroviral therapy: multicentre cohort study. AIDS. 2008 Oct 1;22(15):1943–1950. - PubMed
-
- World Health Organization. Geneva: World Health Organization; 2006. [Accessed November 3, 2008]. Antiretroviral therapy for HIV Infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach. 2006 revision. http://www.who.int/hiv/pub/guidelines/WHO%20Adult%20ART%20Guidelines.pdf.
-
- Delfraissy JF, Flandre P, Delaugerre C, et al. Lopinavir/ritonavir monotherapy or plus zidovudine and lamivudine in antiretroviral-naive HIV-infected patients. AIDS. 2008 Jan 30;22(3):385–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

