Single vector system for efficient N-myristoylation of recombinant proteins in E. coli
- PMID: 20404920
- PMCID: PMC2852408
- DOI: 10.1371/journal.pone.0010081
Single vector system for efficient N-myristoylation of recombinant proteins in E. coli
Abstract
Background: N-myristoylation is a crucial covalent modification of numerous eukaryotic and viral proteins that is catalyzed by N-myristoyltransferase (NMT). Prokaryotes are lacking endogenous NMT activity. Recombinant production of N-myristoylated proteins in E. coli cells can be achieved by coexpression of heterologous NMT with the target protein. In the past, dual plasmid systems were used for this purpose.
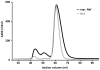
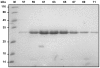
Methodology/principal findings: Here we describe a single vector system for efficient coexpression of substrate and enzyme suitable for production of co- or posttranslationally modified proteins. The approach was validated using the HIV-1 Nef protein as an example. A simple and efficient protocol for production of highly pure and completely N-myristoylated Nef is presented. The yield is about 20 mg myristoylated Nef per liter growth medium.
Conclusions/significance: The single vector strategy allows diverse modifications of target proteins recombinantly coexpressed in E. coli with heterologous enzymes. The method is generally applicable and provides large amounts of quantitatively processed target protein that are sufficient for comprehensive biophysical and structural studies.
Conflict of interest statement
Figures


Similar articles
-
Effects of HIV-1 Nef on human N-myristoyltransferase 1.Biochemistry. 2011 Apr 26;50(16):3394-403. doi: 10.1021/bi200197e. Epub 2011 Mar 30. Biochemistry. 2011. PMID: 21449607 Free PMC article.
-
Protein N-myristoylation in Escherichia coli: reconstitution of a eukaryotic protein modification in bacteria.Proc Natl Acad Sci U S A. 1990 Feb;87(4):1506-10. doi: 10.1073/pnas.87.4.1506. Proc Natl Acad Sci U S A. 1990. PMID: 2406721 Free PMC article.
-
N-Myristoyltransferase isozymes exhibit differential specificity for human immunodeficiency virus type 1 Gag and Nef.J Gen Virol. 2008 Jan;89(Pt 1):288-296. doi: 10.1099/vir.0.83412-0. J Gen Virol. 2008. PMID: 18089753 Free PMC article.
-
Myristoylation.Cell Signal. 1997 Jan;9(1):15-35. doi: 10.1016/s0898-6568(96)00100-3. Cell Signal. 1997. PMID: 9067626 Review.
-
Post-translational myristoylation: Fat matters in cellular life and death.Biochimie. 2011 Jan;93(1):18-31. doi: 10.1016/j.biochi.2010.10.018. Epub 2010 Nov 5. Biochimie. 2011. PMID: 21056615 Review.
Cited by
-
Conformational regulation and target-myristoyl switch of calcineurin B homologous protein 3.Elife. 2023 Jul 12;12:e83868. doi: 10.7554/eLife.83868. Elife. 2023. PMID: 37435805 Free PMC article.
-
N-Lauroylation during the Expression of Recombinant N-Myristoylated Proteins: Implications and Solutions.Chembiochem. 2016 Jan 1;17(1):82-9. doi: 10.1002/cbic.201500454. Epub 2015 Dec 3. Chembiochem. 2016. PMID: 26522884 Free PMC article.
-
N-terminal region of the catalytic domain of human N-myristoyltransferase 1 acts as an inhibitory module.PLoS One. 2015 May 22;10(5):e0127661. doi: 10.1371/journal.pone.0127661. eCollection 2015. PLoS One. 2015. PMID: 26000639 Free PMC article.
-
High yield production of myristoylated Arf6 small GTPase by recombinant N-myristoyl transferase.Small GTPases. 2013 Jan-Mar;4(1):3-8. doi: 10.4161/sgtp.22895. Epub 2013 Jan 1. Small GTPases. 2013. PMID: 23319116 Free PMC article.
-
Effects of HIV-1 Nef on human N-myristoyltransferase 1.Biochemistry. 2011 Apr 26;50(16):3394-403. doi: 10.1021/bi200197e. Epub 2011 Mar 30. Biochemistry. 2011. PMID: 21449607 Free PMC article.
References
-
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. - PubMed
-
- Farazi TA, Waksman G, Gordon JI. The biology and enzymology of protein N-myristoylation. J Biol Chem. 2001;276:39501–39504. - PubMed
-
- Boutin JA. Myristoylation. Cell Signal. 1997;9:15–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

