A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity
- PMID: 20405000
- PMCID: PMC2854128
- DOI: 10.1371/journal.pntd.0000659
A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity
Abstract
Background: The parasitic protozoan Trypanosoma brucei utilizes glycolysis exclusively for ATP production during infection of the mammalian host. The first step in this metabolic pathway is mediated by hexokinase (TbHK), an enzyme essential to the parasite that transfers the gamma-phospho of ATP to a hexose. Here we describe the identification and confirmation of novel small molecule inhibitors of bacterially expressed TbHK1, one of two TbHKs expressed by T. brucei, using a high throughput screening assay.
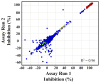
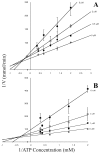
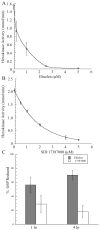
Methodology/principal findings: Exploiting optimized high throughput screening assay procedures, we interrogated 220,233 unique compounds and identified 239 active compounds from which ten small molecules were further characterized. Computation chemical cluster analyses indicated that six compounds were structurally related while the remaining four compounds were classified as unrelated or singletons. All ten compounds were approximately 20-17,000-fold more potent than lonidamine, a previously identified TbHK1 inhibitor. Seven compounds inhibited T. brucei blood stage form parasite growth (0.03<or=EC(50)<3 microM) with parasite specificity of the compounds being demonstrated using insect stage T. brucei parasites, Leishmania promastigotes, and mammalian cell lines. Analysis of two structurally related compounds, ebselen and SID 17387000, revealed that both were mixed inhibitors of TbHK1 with respect to ATP. Additionally, both compounds inhibited parasite lysate-derived HK activity. None of the compounds displayed structural similarity to known hexokinase inhibitors or human African trypanosomiasis therapeutics.
Conclusions/significance: The novel chemotypes identified here could represent leads for future therapeutic development against the African trypanosome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Remme JH, Blas E, Chitsulo L, Desjeux PM, Engers HD, et al. Strategic emphases for tropical diseases research: a TDR perspective. Trends Parasitol. 2002;18:421–426. - PubMed
-
- Pepin J, Milord F. African trypanosomiasis and drug-induced encephalopathy: risk factors and pathogenesis. Trans R Soc Trop Med Hyg. 1991;85:222–224. - PubMed
-
- Albert MA, Haanstra JR, Hannaert V, Van Roy J, Opperdoes FR, et al. Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei. J Biol Chem. 2005;280:28306–28315. - PubMed
-
- Chambers JW, Fowler ML, Morris MT, Morris JC. The anti-trypanosomal agent lonidamine inhibits Trypanosoma brucei hexokinase 1. Mol Biochem Parasitol. 2008;158:202–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

