Maltodextrin: a novel excipient used in sugar-based orally disintegrating tablets and phase transition process
- PMID: 20405257
- PMCID: PMC2902317
- DOI: 10.1208/s12249-010-9423-y
Maltodextrin: a novel excipient used in sugar-based orally disintegrating tablets and phase transition process
Abstract
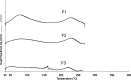
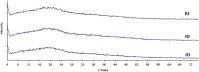
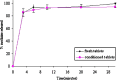
The recent challenge in orally disintegrating tablets (ODT) manufacturing encompasses the compromise between instantaneous disintegration, sufficient hardness, and standard processing equipment. The current investigation constitutes one attempt to fulfill this challenge. Maltodextrin, in the present work, was utilized as a novel excipient to prepare ODT of meclizine. Tablets were prepared by both direct compression and wet granulation techniques. The effect of maltodextrin concentrations on ODT characteristics--manifested as hardness and disintegration time--was studied. The effect of conditioning (40 degrees C and 75% relative humidity) as a post-compression treatment on ODT characteristics was also assessed. Furthermore, maltodextrin-pronounced hardening effect was investigated using differential scanning calorimetry (DSC) and X-ray analysis. Results revealed that in both techniques, rapid disintegration (30-40 s) would be achieved on the cost of tablet hardness (about 1 kg). Post-compression conditioning of tablets resulted in an increase in hardness (3 kg), while keeping rapid disintegration (30-40 s) according to guidance of the FDA for ODT. However, direct compression-conditioning technique exhibited drawbacks of long conditioning time and appearance of the so-called patch effect. These problems were, yet, absent in wet granulation-conditioning technique. DSC and X-ray analysis suggested involvement of glass-elastic deformation in maltodextrin hardening effect. High-performance liquid chromatography analysis of meclizine ODT suggested no degradation of the drug by the applied conditions of temperature and humidity. Overall results proposed that maltodextrin is a promising saccharide for production of ODT with accepted hardness-disintegration time compromise, utilizing standard processing equipment and phenomena of phase transition.
Figures


Similar articles
-
Prediction of quality attributes (mechanical strength, disintegration behavior and drug release) of tablets on the basis of characteristics of granules prepared by high shear wet granulation.PLoS One. 2021 Dec 9;16(12):e0261051. doi: 10.1371/journal.pone.0261051. eCollection 2021. PLoS One. 2021. PMID: 34882723 Free PMC article.
-
Development and characterisation of orally disintegrating flurbiprofen tablets using SeDeM-ODT tool.PLoS One. 2024 Oct 29;19(10):e0309894. doi: 10.1371/journal.pone.0309894. eCollection 2024. PLoS One. 2024. PMID: 39471194 Free PMC article.
-
Potential Use of Magnesium Oxide as an Excipient to Maintain the Hardness of Orally Disintegrating Tablets during Unpackaged Storage.Chem Pharm Bull (Tokyo). 2019;67(3):284-288. doi: 10.1248/cpb.c18-00588. Chem Pharm Bull (Tokyo). 2019. PMID: 30828006
-
Compressed orally disintegrating tablets: excipients evolution and formulation strategies.Expert Opin Drug Deliv. 2013 May;10(5):651-63. doi: 10.1517/17425247.2013.769955. Epub 2013 Feb 7. Expert Opin Drug Deliv. 2013. PMID: 23387409 Review.
-
Challenges and emerging solutions in the development of compressed orally disintegrating tablets.Expert Opin Drug Discov. 2014 Oct;9(10):1109-20. doi: 10.1517/17460441.2014.941802. Epub 2014 Jul 21. Expert Opin Drug Discov. 2014. PMID: 25045997 Review.
Cited by
-
The Use of D-Optimal Mixture Design in Optimizing Development of Okara Tablet Formulation as a Dietary Supplement.ScientificWorldJournal. 2015;2015:684319. doi: 10.1155/2015/684319. Epub 2015 Jun 11. ScientificWorldJournal. 2015. PMID: 26171418 Free PMC article.
-
In Vitro Evaluation of 2D-Printed Edible Films for the Buccal Delivery of Diclofenac Sodium.Materials (Basel). 2018 May 22;11(5):864. doi: 10.3390/ma11050864. Materials (Basel). 2018. PMID: 29789468 Free PMC article.
-
Formulation Development and Characterization of Meclizine Hydrochloride Sublimated Fast Dissolving Tablets.Int Sch Res Notices. 2014 Aug 25;2014:281376. doi: 10.1155/2014/281376. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27355021 Free PMC article.
-
Polycaprolactone/maltodextrin nanocarrier for intracellular drug delivery: formulation, uptake mechanism, internalization kinetics, and subcellular localization.Int J Nanomedicine. 2015 Jul 29;10:4763-81. doi: 10.2147/IJN.S75101. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26251597 Free PMC article.
-
Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota.Neurobiol Stress. 2021 Jan 12;14:100294. doi: 10.1016/j.ynstr.2021.100294. eCollection 2021 May. Neurobiol Stress. 2021. PMID: 33511258 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

