Immobilization of polymer-decorated liquid crystal droplets on chemically tailored surfaces
- PMID: 20405867
- PMCID: PMC2883006
- DOI: 10.1021/la100376u
Immobilization of polymer-decorated liquid crystal droplets on chemically tailored surfaces
Abstract
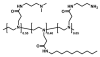
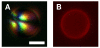
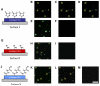
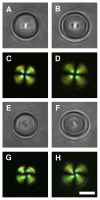
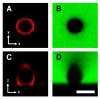
We demonstrate that the assembly of an amphiphilic polyamine on the interfaces of micrometer-sized droplets of a thermotropic liquid crystal (LC) dispersed in aqueous solutions can be used to facilitate the immobilization of LC droplets on chemically functionalized surfaces. Polymer 1 was designed to contain both hydrophobic (alkyl-functionalized) and hydrophilic (primary and tertiary amine-functionalized) side chain functionality. The assembly of this polymer at the interfaces of aqueous dispersions of LC droplets was achieved by the spontaneous adsorption of polymer from aqueous solution. Polymer adsorption triggered transitions in the orientational ordering of the LCs, as observed by polarized light and bright-field microscopy. We demonstrate that the presence of polymer 1 on the interfaces of these droplets can be exploited to immobilize LC droplets on planar solid surfaces through covalent bond formation (e.g., for surfaces coated with polymer multilayers containing reactive azlactone functionality) or through electrostatic interactions (e.g., for surfaces coated with multilayers containing hydrolyzed azlactone functionality). The characterization of immobilized LC droplets by polarized, fluorescence, and laser scanning confocal microscopy revealed the general spherical shape of the polymer-coated LC droplets to be maintained after immobilization, and that immobilization led to additional ordering transitions within the droplets that were dependent on the nature of the surfaces with which they were in contact. Polymer 1-functionalized LC droplets were not immobilized on polymer multilayers treated with poly(ethylene imine) (PEI). We demonstrate that the ability to design surfaces that promote or prevent the immobilization of polymer-functionalized LC droplets can be exploited to pattern the immobilization of LC droplets on surfaces. The results of this investigation provide the basis of an approach that could be used to tailor the properties of dispersed LC emulsions and to immobilize these droplets on functional surfaces of interest in a broad range of fundamental and applied contexts.
Figures



Similar articles
-
Recent advances in colloidal and interfacial phenomena involving liquid crystals.Langmuir. 2011 May 17;27(10):5719-38. doi: 10.1021/la103301d. Epub 2010 Nov 19. Langmuir. 2011. PMID: 21090596 Free PMC article. Review.
-
Covalent Immobilization of Caged Liquid Crystal Microdroplets on Surfaces.ACS Appl Mater Interfaces. 2015 Dec 9;7(48):26892-903. doi: 10.1021/acsami.5b09595. Epub 2015 Nov 24. ACS Appl Mater Interfaces. 2015. PMID: 26562466
-
Dynamic ordering transitions of liquid crystals driven by interfacial complexes formed between polyanions and amphiphilic polyamines.Langmuir. 2008 Dec 2;24(23):13231-6. doi: 10.1021/la803376u. Langmuir. 2008. PMID: 18991416 Free PMC article.
-
Liquid Crystal-Infused Porous Polymer Surfaces: A "Slippery" Soft Material Platform for the Naked-Eye Detection and Discrimination of Amphiphilic Species.ACS Appl Mater Interfaces. 2021 Jul 21;13(28):33652-33663. doi: 10.1021/acsami.1c08170. Epub 2021 Jul 8. ACS Appl Mater Interfaces. 2021. PMID: 34236833 Free PMC article.
-
Optical microscopy studies of dynamics within individual polymer-dispersed liquid crystal droplets.Acc Chem Res. 2005 Feb;38(2):137-45. doi: 10.1021/ar040106p. Acc Chem Res. 2005. PMID: 15709733 Review.
Cited by
-
Azlactone-Functionalized Polymers as Reactive Platforms for the Design of Advanced Materials: Progress in the Last Ten Years.Polym Chem. 2012 Jan 1;3(1):66-80. doi: 10.1039/C1PY00314C. Epub 2011 Oct 12. Polym Chem. 2012. PMID: 29492112 Free PMC article.
-
Fabrication of oligonucleotide and protein arrays on rigid and flexible substrates coated with reactive polymer multilayers.ACS Appl Mater Interfaces. 2013 Jan 23;5(2):351-9. doi: 10.1021/am302285n. Epub 2012 Dec 28. ACS Appl Mater Interfaces. 2013. PMID: 23237360 Free PMC article.
-
Introduction to optical methods for characterizing liquid crystals at interfaces.Langmuir. 2013 Mar 12;29(10):3154-69. doi: 10.1021/la304679f. Epub 2013 Feb 26. Langmuir. 2013. PMID: 23347378 Free PMC article. Review.
-
Recent advances in colloidal and interfacial phenomena involving liquid crystals.Langmuir. 2011 May 17;27(10):5719-38. doi: 10.1021/la103301d. Epub 2010 Nov 19. Langmuir. 2011. PMID: 21090596 Free PMC article. Review.
-
Influence of droplet size, pH and ionic strength on endotoxin-triggered ordering transitions in liquid crystalline droplets.Soft Matter. 2013 Jan 14;9(2):374-382. doi: 10.1039/C2SM26811F. Soft Matter. 2013. PMID: 23675387 Free PMC article.
References
-
- Jerome B. Rep. Prog. Phys. 1991;54:391.
-
- Cognard J. Mol. Cryst. Liq. Cryst. 1982:1.
-
- de Gennes PG, Prost J. The Physics of Liquid Crystals. Oxford University Press; London: 1994.
-
- Brake JM, Daschner MK, Luk YY, Abbott NL. Science. 2003;302:2094. - PubMed
-
- Shah RR, Abbott NL. Science. 2001;293:1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

