Unique physical properties and interactions of the domains of methylated DNA binding protein 2
- PMID: 20405910
- PMCID: PMC2872689
- DOI: 10.1021/bi9019753
Unique physical properties and interactions of the domains of methylated DNA binding protein 2
Abstract
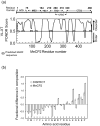
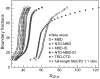
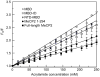
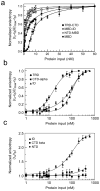
Methylated DNA binding protein 2 (MeCP2) is a methyl CpG binding protein whose key role is the recognition of epigenetic information encoded in DNA methylation patterns. Mutation or misregulation of MeCP2 function leads to Rett syndrome as well as a variety of other autism spectrum disorders. Here, we have analyzed in detail the properties of six individually expressed human MeCP2 domains spanning the entire protein with emphasis on their interactions with each other, with DNA, and with nucleosomal arrays. Each domain contributes uniquely to the structure and function of the full-length protein. MeCP2 is approximately 60% unstructured, with nine interspersed alpha-molecular recognition features (alpha-MoRFs), which are polypeptide segments predicted to acquire secondary structure upon forming complexes with binding partners. Large increases in secondary structure content are induced in some of the isolated MeCP2 domains and in the full-length protein by binding to DNA. Interactions between some MeCP2 domains in cis and trans seen in our assays likely contribute to the structure and function of the intact protein. We also show that MeCP2 has two functional halves. The N-terminal portion contains the methylated DNA binding domain (MBD) and two highly disordered flanking domains that modulate MBD-mediated DNA binding. One of these flanking domains is also capable of autonomous DNA binding. In contrast, the C-terminal portion of the protein that harbors at least two independent DNA binding domains and a chromatin-specific binding domain is largely responsible for mediating nucleosomal array compaction and oligomerization. These findings led to new mechanistic and biochemical insights regarding the conformational modulations of this intrinsically disordered protein, and its context-dependent in vivo roles.
Figures




Similar articles
-
Influence of the disordered domain structure of MeCP2 on its structural stability and dsDNA interaction.Int J Biol Macromol. 2021 Apr 1;175:58-66. doi: 10.1016/j.ijbiomac.2021.01.206. Epub 2021 Feb 3. Int J Biol Macromol. 2021. PMID: 33548325
-
The intervening domain from MeCP2 enhances the DNA affinity of the methyl binding domain and provides an independent DNA interaction site.Sci Rep. 2017 Jan 31;7:41635. doi: 10.1038/srep41635. Sci Rep. 2017. PMID: 28139759 Free PMC article.
-
Binding of the Rett syndrome protein, MeCP2, to methylated and unmethylated DNA and chromatin.IUBMB Life. 2010 Oct;62(10):732-8. doi: 10.1002/iub.386. IUBMB Life. 2010. PMID: 21031501 Free PMC article. Review.
-
Multiple modes of interaction between the methylated DNA binding protein MeCP2 and chromatin.Mol Cell Biol. 2007 Feb;27(3):864-77. doi: 10.1128/MCB.01593-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101771 Free PMC article.
-
MeCP2: the long trip from a chromatin protein to neurological disorders.Trends Mol Med. 2014 Sep;20(9):487-98. doi: 10.1016/j.molmed.2014.03.004. Epub 2014 Apr 21. Trends Mol Med. 2014. PMID: 24766768 Review.
Cited by
-
The Molecular Functions of MeCP2 in Rett Syndrome Pathology.Front Genet. 2021 Apr 23;12:624290. doi: 10.3389/fgene.2021.624290. eCollection 2021. Front Genet. 2021. PMID: 33968128 Free PMC article. Review.
-
Impaired in vivo binding of MeCP2 to chromatin in the absence of its DNA methyl-binding domain.Nucleic Acids Res. 2013 May;41(9):4888-900. doi: 10.1093/nar/gkt213. Epub 2013 Apr 4. Nucleic Acids Res. 2013. PMID: 23558747 Free PMC article.
-
Chromatin context and ncRNA highlight targets of MeCP2 in brain.RNA Biol. 2013 Nov;10(11):1741-57. doi: 10.4161/rna.26921. Epub 2013 Oct 30. RNA Biol. 2013. PMID: 24270455 Free PMC article.
-
Disease mutations in disordered regions--exception to the rule?Mol Biosyst. 2012 Jan;8(1):27-32. doi: 10.1039/c1mb05251a. Epub 2011 Nov 14. Mol Biosyst. 2012. PMID: 22080206 Free PMC article. Review.
-
An AT-hook domain in MeCP2 determines the clinical course of Rett syndrome and related disorders.Cell. 2013 Feb 28;152(5):984-96. doi: 10.1016/j.cell.2013.01.038. Cell. 2013. PMID: 23452848 Free PMC article.
References
-
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:91–97. - PubMed
-
- Amir RE, den Veyber IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MeCP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
-
- LaSalle JM, Hogart A, Thatcher KN. Rett syndrome: a Rosetta stone for understanding the molecular pathogenesis of autism. Int Rev Neurobiol. 2005;71:131–165. - PubMed
-
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr Opin Genet Dev. 2006;16:276–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM066834/GM/NIGMS NIH HHS/United States
- R01 LM007688/LM/NLM NIH HHS/United States
- GM070897/GM/NIGMS NIH HHS/United States
- DP1 OD000945/OD/NIH HHS/United States
- OD945/OD/NIH HHS/United States
- LM007688/LM/NLM NIH HHS/United States
- R01 GM027616/GM/NIGMS NIH HHS/United States
- GM027616/GM/NIGMS NIH HHS/United States
- GM066834/GM/NIGMS NIH HHS/United States
- R01 GM070897/GM/NIGMS NIH HHS/United States
- GM071714/GM/NIGMS NIH HHS/United States
- R01 GM071714/GM/NIGMS NIH HHS/United States
- R56 LM007688/LM/NLM NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

