Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies
- PMID: 20406068
- PMCID: PMC2905054
- DOI: 10.2217/bmm.10.1
Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies
Abstract
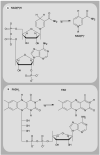
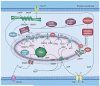
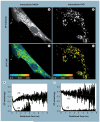
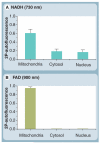
Mitochondria play a pivotal role in energy metabolism, programmed cell death and oxidative stress. Mutated mitochondrial DNA in diseased cells compromises the structure of key enzyme complexes and, therefore, mitochondrial function, which leads to a myriad of health-related conditions such as cancer, neurodegenerative diseases, diabetes and aging. Early detection of mitochondrial and metabolic anomalies is an essential step towards effective diagnoses and therapeutic intervention. Reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) play important roles in a wide range of cellular oxidation-reduction reactions. Importantly, NADH and FAD are naturally fluorescent, which allows noninvasive imaging of metabolic activities of living cells and tissues. Furthermore, NADH and FAD autofluorescence, which can be excited using distinct wavelengths for complementary imaging methods and is sensitive to protein binding and local environment. This article highlights recent developments concerning intracellular NADH and FAD as potential biomarkers for metabolic and mitochondrial activities.
Figures


References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th Edition Garland Science; NY, USA: 2002.
-
- Christen Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71(2):621S–629S. - PubMed
-
- Jacquard C, Trioulier Y, Cosker F, et al. Brain mitochondrial defects amplify intracellular [Ca2+] rise and neurodegeneration but not Ca2+ entry during NMDA receptor activation. FASEB J. 2006;20(7):1021–1023. - PubMed
-
-
Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 2004;25(4):365–451. ▪▪ Insightful perspective on mitochondria in health, diseases and basic biology.
-
-
- Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl. 1):S96–S102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
