Impaired metabolic effects of a thyroid hormone receptor beta-selective agonist in a mouse model of diet-induced obesity
- PMID: 20406106
- PMCID: PMC2941403
- DOI: 10.1089/thy.2009.0318
Impaired metabolic effects of a thyroid hormone receptor beta-selective agonist in a mouse model of diet-induced obesity
Abstract
Background: The use of selective agonists of the thyroid hormone receptor isoform beta (TRbeta) has been linked to metabolic improvement in animal models of diet-induced obesity, nonalcoholic liver disease, and genetic hypercholesterolemia.
Methods: To identify potential target tissues of such compounds, we exposed primary murine brown adipocytes and skeletal myocytes for 24 hours to 50 nM GC-24, a highly selective TRbeta agonist. GC-24 (17 ng/[g BW.day] for 36 days) was also tested in a mouse model of diet-induced obesity.
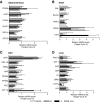
Results: While the brown adipocytes responded to GC-24, with 17%-400% increases in the expression of 12 metabolically relevant genes, the myocytes remained largely unresponsive to GC-24 treatment. In control mice kept on chow diet, GC-24 treatment accelerated energy expenditure by about 15% and limited body weight gain by about 50%. However, in the obese animals the GC-24-mediated reduction in body weight gain dropped to only 20%, while energy expenditure remained unaffected. In addition, an analysis of gene expression in the skeletal muscle, brown adipose tissue, and liver of these obese animals failed to identify a conclusive GC-24 transcriptome footprint.
Conclusion: Feeding a high-fat diet impairs most of the beneficial metabolic effects associated with treatment with TRbeta-selective agonists.
Figures




Comment in
-
Thyroid hormone actions: to Beta or not to Beta.Thyroid. 2010 May;20(5):451-2. doi: 10.1089/thy.2010.1630. Thyroid. 2010. PMID: 20450428 No abstract available.
Similar articles
-
A TRbeta-selective agonist confers resistance to diet-induced obesity.J Endocrinol. 2009 Nov;203(2):291-9. doi: 10.1677/JOE-08-0539. Epub 2009 Aug 27. J Endocrinol. 2009. PMID: 19713219 Free PMC article.
-
Sustained zero-order delivery of GC-1 from a nanochannel membrane device alleviates metabolic syndrome.Int J Obes (Lond). 2016 Nov;40(11):1776-1783. doi: 10.1038/ijo.2016.129. Epub 2016 Jul 27. Int J Obes (Lond). 2016. PMID: 27460601
-
PPAR-α agonist elicits metabolically active brown adipocytes and weight loss in diet-induced obese mice.Cell Biochem Funct. 2015 Jun;33(4):249-56. doi: 10.1002/cbf.3111. Epub 2015 May 11. Cell Biochem Funct. 2015. PMID: 25959716
-
Brown adipose tissue: a potential target in the fight against obesity and the metabolic syndrome.Clin Sci (Lond). 2015 Dec;129(11):933-49. doi: 10.1042/CS20150339. Clin Sci (Lond). 2015. PMID: 26359253 Review.
-
Therapeutic potential for thyroid hormone receptor-beta selective agonists for treating obesity, hyperlipidemia and diabetes.Curr Vasc Pharmacol. 2007 Apr;5(2):141-54. doi: 10.2174/157016107780368271. Curr Vasc Pharmacol. 2007. PMID: 17430219 Review.
Cited by
-
Dexamethasone reduces energy expenditure and increases susceptibility to diet-induced obesity in mice.Obesity (Silver Spring). 2013 Sep;21(9):E415-20. doi: 10.1002/oby.20338. Obesity (Silver Spring). 2013. PMID: 23408649 Free PMC article.
-
Emerging Therapies in Hypothyroidism.Annu Rev Med. 2024 Jan 29;75:307-319. doi: 10.1146/annurev-med-060622-101007. Epub 2023 Sep 22. Annu Rev Med. 2024. PMID: 37738506 Free PMC article. Review.
-
Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism.Endocrinology. 2010 Dec;151(12):5952-60. doi: 10.1210/en.2010-0631. Epub 2010 Sep 29. Endocrinology. 2010. PMID: 20881246 Free PMC article.
-
Pharmacological modulation of adaptive thermogenesis: new clues for obesity management?J Endocrinol Invest. 2023 Nov;46(11):2213-2236. doi: 10.1007/s40618-023-02125-0. Epub 2023 Jun 28. J Endocrinol Invest. 2023. PMID: 37378828 Free PMC article. Review.
-
Paradigms of Dynamic Control of Thyroid Hormone Signaling.Endocr Rev. 2019 Aug 1;40(4):1000-1047. doi: 10.1210/er.2018-00275. Endocr Rev. 2019. PMID: 31033998 Free PMC article. Review.
References
-
- Bianco AC. Maia AL. da Silva WS. Christoffolete MA. Adaptive activation of thyroid hormone and energy expenditure. Biosci Rep. 2005;25:191–208. - PubMed
-
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. - PubMed
-
- Brent GA. Tissue-specific actions of thyroid hormone: insights from animal models. Rev Endocr Metab Disord. 2000;1:27–33. - PubMed
-
- Chiellini G. Apriletti JW. Yoshihara HA. Baxter JD. Ribeiro RC. Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5:299–306. - PubMed
-
- Trost SU. Swanson E. Gloss B. Wang-Iverson DB. Zhang H. Volodarsky T. Grover GJ. Baxter JD. Chiellini G. Scanlan TS. Dillmann WH. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141:3057–3064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

