Translational pharmacokinetic-pharmacodynamic modelling; application to cardiovascular safety data for PF-00821385, a novel HIV agent
- PMID: 20406218
- PMCID: PMC2848407
- DOI: 10.1111/j.1365-2125.2009.03594.x
Translational pharmacokinetic-pharmacodynamic modelling; application to cardiovascular safety data for PF-00821385, a novel HIV agent
Abstract
Aim: To assess the translation of pharmacokinetic-pharmacodynamic (PK-PD) relationships for heart rate effects of PF-00821385 in dog and man.
Methods: Cardiovascular telemetric parameters and concentration data were available for animals receiving active doses (0.5-120 mg kg(-1), n= 4) or vehicle. PF-00821385 was administered to 24 volunteers and pharmacokinetic and vital signs data were collected. PK-PD models were fitted using nonlinear mixed effects.
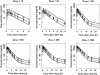
Results: Compartmental models with linear absorption and clearance were used to describe pharmacokinetic disposition in animal and man. Diurnal variation in heart and pulse rate was best described with a single cosine function in both dog and man. Canine and human heart rate change were described by a linear model with free drug slope 1.76 bpm microM(-1)[95% confidence interval (CI) 1.17, 2.35] in the dog and 0.76 bpm microM(-1) (95% CI 0.54, 1.14) in man.
Conclusions: The preclinical translational of concentration-response has been described and the potential for further interspecies extrapolation and optimization of clinical trial design is addressed.
Figures




Similar articles
-
Translational and pharmacokinetic-pharmacodynamic application for the clinical development of GDC-0334, a novel TRPA1 inhibitor.Clin Transl Sci. 2021 Sep;14(5):1945-1954. doi: 10.1111/cts.13049. Epub 2021 May 31. Clin Transl Sci. 2021. PMID: 34058071 Free PMC article. Clinical Trial.
-
Translational PK/PD modeling for cardiovascular safety assessment of drug candidates: Methods and examples in drug development.J Pharmacol Toxicol Methods. 2014 Jul-Aug;70(1):73-85. doi: 10.1016/j.vascn.2014.05.004. Epub 2014 May 28. J Pharmacol Toxicol Methods. 2014. PMID: 24879942
-
Maraviroc modelling strategy: use of early phase 1 data to support a semi-mechanistic population pharmacokinetic model.Br J Clin Pharmacol. 2009 Sep;68(3):355-69. doi: 10.1111/j.1365-2125.2009.03455.x. Br J Clin Pharmacol. 2009. PMID: 19740392 Free PMC article.
-
Integration and modelling of pharmacokinetic and pharmacodynamic data to optimize dosage regimens in veterinary medicine.J Vet Pharmacol Ther. 2004 Dec;27(6):467-77. doi: 10.1111/j.1365-2885.2004.00613.x. J Vet Pharmacol Ther. 2004. PMID: 15601441 Review.
-
Clinical safety and pharmacology trial.Curr Top Microbiol Immunol. 2014;383:79-95. doi: 10.1007/82_2013_355. Curr Top Microbiol Immunol. 2014. PMID: 24173586 Review.
Cited by
-
Systems pharmacology: bridging systems biology and pharmacokinetics-pharmacodynamics (PKPD) in drug discovery and development.Pharm Res. 2011 Jul;28(7):1460-4. doi: 10.1007/s11095-011-0467-9. Epub 2011 May 11. Pharm Res. 2011. PMID: 21560018
-
A tutorial for model-based evaluation and translation of cardiovascular safety in preclinical trials.CPT Pharmacometrics Syst Pharmacol. 2024 Jan;13(1):5-22. doi: 10.1002/psp4.13082. Epub 2023 Nov 23. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 37950388 Free PMC article.
-
Pharmacokinetic-pharmacodynamic modeling of alpha interferon response induced by a Toll-like 7 receptor agonist in mice.Antimicrob Agents Chemother. 2010 Mar;54(3):1179-85. doi: 10.1128/AAC.00551-09. Epub 2009 Dec 22. Antimicrob Agents Chemother. 2010. PMID: 20028817 Free PMC article.
-
The role of concentration-effect relationships in the assessment of QTc interval prolongation.Br J Clin Pharmacol. 2015 Jan;79(1):117-31. doi: 10.1111/bcp.12443. Br J Clin Pharmacol. 2015. PMID: 24938719 Free PMC article. Review.
-
Modeling and Simulation Approaches for Cardiovascular Function and Their Role in Safety Assessment.CPT Pharmacometrics Syst Pharmacol. 2015 Mar;4(3):e00018. doi: 10.1002/psp4.18. Epub 2015 Mar 11. CPT Pharmacometrics Syst Pharmacol. 2015. PMID: 26225237 Free PMC article. Review.
References
-
- Poignard P, Saphire EO, Parren PW, Burton DR. gp120: biological aspects of structural features. Annu Rev Immunol. 2001;19:253–74. - PubMed
-
- Danhof M, de Lange EC, Della Pasqua OE, Ploeger BA, Voskuyl RA. Mechanism-based pharmacokinetic–pharmacodynamic (PK–PD) modeling in translational drug research. Trends Pharmacol Sci. 2008;29:186–91. - PubMed
-
- Beal SL, Boeckmann AJ. San Francisco, CA: University of California; NONMEM Users Guides. 1989–1998.
-
- Jonsson EN, Karlsson MO. Xpose – an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64. - PubMed
-
- Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit – a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

