Dopamine and glutamate in Huntington's disease: A balancing act
- PMID: 20406248
- PMCID: PMC3118459
- DOI: 10.1111/j.1755-5949.2010.00134.x
Dopamine and glutamate in Huntington's disease: A balancing act
Abstract
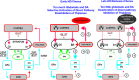
Huntington's disease (HD) is caused by a CAG repeat expansion in exon 1 of the HD gene resulting in a long polyglutamine tract in the N-terminus of the protein huntingtin. Patients carrying the mutation display chorea in early stages followed by akinesia and sometimes dystonia in late stages. Other major symptoms include depression, anxiety, irritability or aggressive behavior, and apathy. Although many neuronal systems are affected, dysfunction and subsequent neurodegeneration in the basal ganglia and cortex are the most apparent pathologies. In HD, the primary hypothesis has been that there is an initial overactivity of glutamate neurotransmission that produces excitotoxicity followed by a series of complex changes that are different in the striatum and in the cortex. This review will focus on evidence for alterations in dopamine (DA)-glutamate interactions in HD, concentrating on the striatum and cortex. The most recent evidence points to decreases in DA and glutamate neurotransmission as the HD phenotype develops. However, there is some evidence for increased DA and glutamate functions that could be responsible for some of the early HD phenotype. Significant evidence indicates that glutamate and dopamine neurotransmission is affected in HD, compromising the fine balance in which DA modulates glutamate-induced excitation in the basal ganglia and cortex. Restoring the balance between glutamate and dopamine could be helpful to treat HD symptoms.
Conflict of interest statement
The authors have no conflict of interest.
Figures
References
-
- The Huntington's Disease Collaborative Research Group . A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 1993;72:971–983. - PubMed
-
- Bonelli RM, Hofmann P. A systematic review of the treatment studies in Huntington's disease since 1990. Expert Opin Pharmacother 2007;8:141–153. - PubMed
-
- Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature 1976;263:244–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

