Why has the antidepressant-placebo difference in antidepressant clinical trials diminished over the past three decades?
- PMID: 20406269
- PMCID: PMC6493812
- DOI: 10.1111/j.1755-5949.2010.00151.x
Why has the antidepressant-placebo difference in antidepressant clinical trials diminished over the past three decades?
Abstract
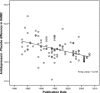
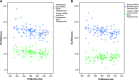
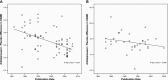
The increasing rate of failure of antidepressant clinical trials has led to the assertion that antidepressants do not have meaningful clinical benefits. Our hypothesis was that the decrease in antidepressant-placebo differences in antidepressant clinical trials over the past three decades could be explained by changes in research design features rather than a lack of potency of the antidepressants being tested. We collected data from 130 double blind placebo controlled antidepressant clinical trials conducted between 1981 and 2008 that included 35,122 depressed patients with 23,157 patients assigned to antidepressants and 11,965 assigned to placebo. We conducted a hierarchical regression analysis of change in HAM-D scores in antidepressant and placebo groups separately with year of publication, and research design features as independent variables. We found that antidepressant-placebo differences in antidepressant clinical trials have declined markedly over the past three decades. Decline in change scores in the antidepressant group was related to mean total baseline HAM-D scores in the trial, the version of HAM-D used, and duration of trial. Similarly, decline in change scores in the placebo group was related to mean total baseline HAM-D scores, duration of trial, and year of publication. Overall, we found that antidepressant-placebo differences were statistically significantly higher in trials that used HAM-D 21 rather than HAM-D 17 and in trials that lasted 6 weeks or less. These data suggest that, apart from the efficacy of the antidepressant being tested, factors such as baseline HAM-D scores, version of HAM-D used and duration of trial have a significant impact on outcome. As such a clinician's assessment of the usefulness of antidepressants should not be based solely on the results of such clinical trials. In the meantime there is a need for continuing research to improve the methodology of antidepressant clinical trials. These data suggest that many aspects of the design of antidepressant trials have a significant impact on outcome. Further, these data suggest that the results of more recent placebo controlled trials do not adequately inform clinicians about the potential utility of antidepressants.
Figures
References
-
- Broich K. Committee for medicinal products for human use (CHMP) assessment on efficacy of antidepressants. Eur Neuropsychopharmacol 2009;19:305–308. - PubMed
-
- Walsh BT, Seidman S, Sysko R, et al Placebo response in studies of major depression: Variable, substantial, and growing. JAMA 2002;287:840–1847. - PubMed
-
- Khan A, Leventhal S, Khan S, et al Severity of depression and response to antidepressants and placebo: An analysis of the FDA database. J Clin Psychopharmacol 2002;22:40–45. - PubMed
-
- Khan A, Kolts RL, Thase ME, et al Research design features and patient characteristics associated with the outcome of antidepressant clinical trials. Am J Psychiatry 2004;161:2045–2049. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

