The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1)
- PMID: 20406299
- PMCID: PMC2966764
- DOI: 10.1111/j.1365-2567.2010.03281.x
The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1)
Abstract
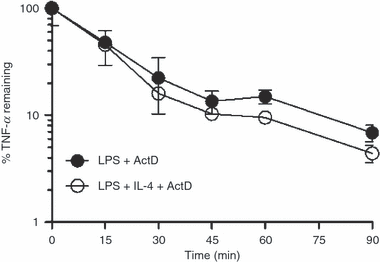
While it is known that the anti-inflammatory effects of interleukin (IL)-4 require new protein synthesis, the exact mechanisms by which IL-4 suppresses the production of pro-inflammatory cytokines by human monocytes and macrophages is unclear. IL-4 rapidly induced suppressor of cytokine signalling-1 (SOCS1) mRNA and protein, which peaked at 60 min, much earlier than lipopolysaccharide (LPS)-induced SOCS1 mRNA and protein which were consistently maximal 4 hr post-exposure. SOCS1 is a molecule generally considered to be induced for negative feedback of inflammatory processes. We investigated whether the early induction of SOCS1 by IL-4 was responsible for the suppression of LPS-induced tumour necrosis factor (TNF)-alpha production by IL-4. IL-4 suppressed LPS-induced TNF-alpha in freshly isolated monocytes at the level of transcription but acted by a different, possibly translational, mechanism in monocytes cultured overnight in macrophage colony-stimulating factor (M-CSF). Despite different modes of regulation by IL-4, the kinetics and magnitude of induction of SOCS1 mRNA and protein by IL-4 in the two cell types were identical. There was no significant difference in the suppression by IL-4 of LPS-induced TNF-alpha production by bone-marrow derived macrophages from wild-type mice, Ifngamma(-/-) mice and mice lacking SOCS1 (Socs1(-/-)Ifngamma(-/-)). These data suggest that SOCS1 is not involved in the suppression of LPS-induced TNF-alpha production by IL-4.
Figures






Similar articles
-
SOCS1 regulates the IFN but not NFkappaB pathway in TLR-stimulated human monocytes and macrophages.J Immunol. 2008 Dec 1;181(11):8018-26. doi: 10.4049/jimmunol.181.11.8018. J Immunol. 2008. PMID: 19017994 Free PMC article.
-
Suppressor of cytokine signalling-3 at pathological levels does not regulate lipopolysaccharide or interleukin-10 control of tumour necrosis factor-alpha production by human monocytes.Immunology. 2006 Sep;119(1):8-17. doi: 10.1111/j.1365-2567.2006.02383.x. Immunology. 2006. PMID: 16925527 Free PMC article.
-
Muramyldipeptide augments the actions of lipopolysaccharide in mice by stimulating macrophages to produce pro-IL-1β and by down-regulation of the suppressor of cytokine signaling 1 (SOCS1).Innate Immun. 2011 Feb;17(1):3-15. doi: 10.1177/1753425909347508. Epub 2009 Nov 6. Innate Immun. 2011. PMID: 19897531
-
The comparative roles of suppressor of cytokine signaling-1 and -3 in the inhibition and desensitization of cytokine signaling.J Biol Chem. 2006 Apr 21;281(16):11135-43. doi: 10.1074/jbc.M509595200. Epub 2006 Feb 10. J Biol Chem. 2006. PMID: 16473883
-
Regulation of chemokine expression by antiinflammatory cytokines.Immunol Res. 2002;25(3):229-45. doi: 10.1385/IR:25:3:229. Immunol Res. 2002. PMID: 12018462 Review.
Cited by
-
The Implication of Mechanistic Approaches and the Role of the Microbiome in Polycystic Ovary Syndrome (PCOS): A Review.Metabolites. 2023 Jan 14;13(1):129. doi: 10.3390/metabo13010129. Metabolites. 2023. PMID: 36677054 Free PMC article. Review.
-
Improving the Function and Engraftment of Transplanted Pancreatic Islets Using Pulsed Focused Ultrasound Therapy.Sci Rep. 2019 Sep 16;9(1):13416. doi: 10.1038/s41598-019-49933-0. Sci Rep. 2019. PMID: 31527773 Free PMC article.
-
Soft Tissue Manipulation Alters RANTES/CCL5 and IL-4 Cytokine Levels in a Rat Model of Chronic Low Back Pain.Int J Mol Sci. 2023 Sep 21;24(18):14392. doi: 10.3390/ijms241814392. Int J Mol Sci. 2023. PMID: 37762698 Free PMC article.
-
Biomarkers of Postpartum Depression: A Narrative Review.J Clin Med. 2023 Oct 14;12(20):6519. doi: 10.3390/jcm12206519. J Clin Med. 2023. PMID: 37892657 Free PMC article. Review.
-
Of Mice and Men: Comparative Analysis of Neuro-Inflammatory Mechanisms in Human and Mouse Using Cause-and-Effect Models.J Alzheimers Dis. 2017;59(3):1045-1055. doi: 10.3233/JAD-170255. J Alzheimers Dis. 2017. PMID: 28731442 Free PMC article. Review.
References
-
- Fenton MJ, Buras JA, Donnelly RP. IL-4 reciprocally regulates IL-1 and IL-1 receptor antagonist expression in human monocytes. J Immunol. 1992;15:1283–8. - PubMed
-
- Colotta F, Re F, Muzio M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;23:472–5. - PubMed
-
- Cawston TE, Ellis AJ, Bigg H, Curry V, Lean E, Ward D. Interleukin-4 blocks the release of collagen fragments from bovine nasal cartilage treated with cytokines. Biochim Biophys Acta. 1996;1314:226–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

