Histamine-treated dendritic cells improve recruitment of type 2 CD8 T cells in the lungs of allergic mice
- PMID: 20406304
- PMCID: PMC2913269
- DOI: 10.1111/j.1365-2567.2010.03262.x
Histamine-treated dendritic cells improve recruitment of type 2 CD8 T cells in the lungs of allergic mice
Abstract
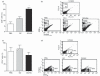
Histamine controls the function of dendritic cells (DCs). It appears to be required for the normal development of DCs. It also induces the chemotaxis of immature DCs and promotes the differentiation of CD4(+) T cells into cells with a T helper type 2 (Th2) profile. Moreover, we have recently shown that histamine stimulates both the uptake and the cross-presentation of antigens by DCs, supporting the theory that histamine promotes activation of CD8(+) T cells during the development of allergic pathologies. Here, we investigated whether the course of an allergic response, in a well-defined model of ovalbumin (OVA)-induced allergic airway inflammation, could be modulated by intratracheal injection of OVA-pulsed DCs previously treated with histamine (DCHISs). Compared with control DCs, DCHISs induced: (i) greater recruitment of CD8(+) T cells in the lung, (ii) greater stimulation of the production of interleukin (IL)-5 by lung CD8(+) T cells, and (iii) increased recruitment of CD11c/CD8 double-positive DCs in the lungs of allergic mice. Moreover, mice treated with DCHISs showed increased levels of serum-specific immunoglobulin E (IgE) antibodies directed to OVA, and a higher proportion of eosinophils in bronchoalveolar lavage (BAL) compared with mice treated with OVA-pulsed control DCs. Our results support the notion that histamine, by acting on DCs, increases the severity of allergic processes.
Figures

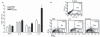
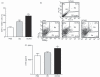
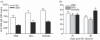
Similar articles
-
Fms-like tyrosine kinase 3 ligand regulates migratory pattern and antigen uptake of lung dendritic cell subsets in a murine model of allergic airway inflammation.J Immunol. 2009 Dec 1;183(11):7531-8. doi: 10.4049/jimmunol.0901341. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917684
-
Histamine improves antigen uptake and cross-presentation by dendritic cells.J Immunol. 2007 Sep 15;179(6):3425-33. doi: 10.4049/jimmunol.179.6.3425. J Immunol. 2007. PMID: 17785776
-
Derp1-modified dendritic cells attenuate allergic inflammation by regulating the development of T helper type1(Th1)/Th2 cells and regulatory T cells in a murine model of allergic rhinitis.Mol Immunol. 2017 Oct;90:172-181. doi: 10.1016/j.molimm.2017.07.015. Epub 2017 Aug 9. Mol Immunol. 2017. PMID: 28802126
-
Differential capacity of CD8+ alpha or CD8- alpha dendritic cell subsets to prime for eosinophilic airway inflammation in the T-helper type 2-prone milieu of the lung.Clin Exp Allergy. 2004 Dec;34(12):1834-40. doi: 10.1111/j.1365-2222.2004.02133.x. Clin Exp Allergy. 2004. PMID: 15663556
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
Cited by
-
Modulation of Dendritic Cell Apoptosis and CD8+ Cytotoxicity by Histamine: Role of Protein Kinase C.Mediators Inflamm. 2017;2017:9402814. doi: 10.1155/2017/9402814. Epub 2017 Aug 29. Mediators Inflamm. 2017. PMID: 28947859 Free PMC article.
-
Mechanisms of unconventional CD8 Tc2 lymphocyte induction in allergic contact dermatitis: Role of H3/H4 histamine receptors.Front Immunol. 2022 Oct 7;13:999852. doi: 10.3389/fimmu.2022.999852. eCollection 2022. Front Immunol. 2022. PMID: 36275674 Free PMC article.
-
Thioperamide induces CD4 CD25 Foxp3 regulatory T lymphocytes in the lung mucosa of allergic mice through its action on dendritic cells.J Asthma Allergy. 2011;4:93-102. doi: 10.2147/JAA.S23507. Epub 2011 Sep 26. J Asthma Allergy. 2011. PMID: 22034573 Free PMC article.
-
Antagonism of histamine H4 receptors exacerbates clinical and pathological signs of experimental autoimmune encephalomyelitis.Br J Pharmacol. 2013 Sep;170(1):67-77. doi: 10.1111/bph.12263. Br J Pharmacol. 2013. PMID: 23735232 Free PMC article.
-
Regulation of eosinophil trafficking by SWAP-70 and its role in allergic airway inflammation.J Immunol. 2012 Feb 1;188(3):1479-90. doi: 10.4049/jimmunol.1102253. Epub 2011 Dec 30. J Immunol. 2012. PMID: 22210919 Free PMC article.
References
-
- Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;32:2325–30. - PubMed
-
- Worsley AG, LeibundGut-Landmann S, Slack E, Phng LK, Gerhardt H, Reis e Sousa C, MacDonald AS. Dendritic cell expression of the Notch ligand jagged2 is not essential for Th2 response induction in vivo. Eur J Immunol. 2008;38:1043–9. - PubMed
-
- Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. - PubMed
-
- MacDonald AS, Straw AD, Dalton NM, Pearce EJ. Cutting edge: Th2 response induction by dendritic cells: a role for CD40. J Immunol. 2002;168:537–40. - PubMed
-
- Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA. Immune regulation by histamine. Curr Opin Immunol. 2002;14:735–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

