In vivo imaging of CT26 mouse tumours by using cmHsp70.1 monoclonal antibody
- PMID: 20406322
- PMCID: PMC3922674
- DOI: 10.1111/j.1582-4934.2010.01067.x
In vivo imaging of CT26 mouse tumours by using cmHsp70.1 monoclonal antibody
Abstract
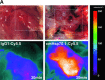
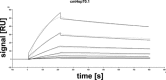
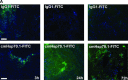
The major stress-inducible heat shock protein 70 (Hsp70) is frequently present on the cell surface of human tumours, but not on normal cells. Herein, the binding characteristics of the cmHsp70.1 mouse monoclonal antibody (mAb) were evaluated in vitro and in a syngeneic tumour mouse model. More than 50% of the CT26 mouse colon carcinoma cells express Hsp70 on their cell surface at 4°C. After a temperature shift to 37°C, the cmHsp70.1-fluorescein isothiocyanate mAb translocates into early endosomes and lysosomes. Intraoperative and near-infrared fluorescence imaging revealed an enrichment of Cy5.5-conjugated mAb cmHsp70.1, but not an identically labelled IgG1 isotype-matched control, in i.p. and s.c. located CT26 tumours, as soon as 30 min. after i.v. injection into the tail vein. Due to the rapid turnover rate of membrane-bound Hsp70, the fluorescence-labelled cmHsp70.1 mAb became endocytosed and accumulated in the tumour, reaching a maximum after 24 hrs and remained detectable at least up to 96 hrs after a single i.v. injection. The tumour-selective internalization of mAb cmHsp70.1 at the physiological temperature of 37°C might enable a targeted uptake of toxins or radionuclides into Hsp70 membrane-positive tumours. The anti-tumoral activity of the cmHsp70.1 mAb is further supported by its capacity to mediate antibody-dependent cytotoxicity.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
















References
-
- Ferrarini M, Heltai S, Zocchi MR, et al. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613–9. - PubMed
-
- Shin BK, Wang H, Yim AM, et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–16. - PubMed
-
- Multhoff G, Botzler C, Wiesnet M, et al. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

