Gliadin fragments promote migration of dendritic cells
- PMID: 20406323
- PMCID: PMC3922678
- DOI: 10.1111/j.1582-4934.2010.01066.x
Gliadin fragments promote migration of dendritic cells
Abstract
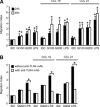
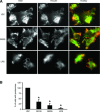
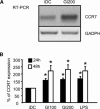
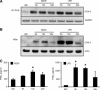
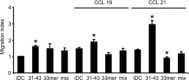
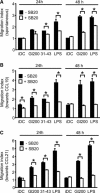
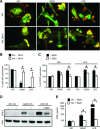
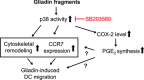
In genetically predisposed individuals, ingestion of wheat gliadin provokes a T-cell-mediated enteropathy, celiac disease. Gliadin fragments were previously reported to induce phenotypic maturation and Th1 cytokine production by human dendritic cells (DCs) and to boost their capacity to stimulate allogeneic T cells. Here, we monitor the effects of gliadin on migratory capacities of DCs. Using transwell assays, we show that gliadin peptic digest stimulates migration of human DCs and their chemotactic responsiveness to the lymph node-homing chemokines CCL19 and CCL21. The gliadin-induced migration is accompanied by extensive alterations of the cytoskeletal organization, with dissolution of adhesion structures, podosomes, as well as up-regulation of the CC chemokine receptor (CCR) 7 on cell surface and induction of cyclooxygenase (COX)-2 enzyme that mediates prostaglandin E2 (PGE₂) production. Blocking experiments confirmed that gliadin-induced migration is independent of the TLR4 signalling. Moreover, we showed that the α-gliadin-derived 31-43 peptide is an active migration-inducing component of the digest. The migration promoted by gliadin fragments or the 31-43 peptide required activation of p38 mitogen-activated protein kinase (MAPK). As revealed using p38 MAPK inhibitor SB203580, this was responsible for DC cytoskeletal transition, CCR7 up-regulation and PGE₂ production in particular. Taken together, this study provides a new insight into pathogenic features of gliadin fragments by demonstrating their ability to promote DC migration, which is a prerequisite for efficient priming of naive T cells, contributing to celiac disease pathology.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
Similar articles
-
Gliadin fragments induce phenotypic and functional maturation of human dendritic cells.J Immunol. 2005 Nov 15;175(10):7038-45. doi: 10.4049/jimmunol.175.10.7038. J Immunol. 2005. PMID: 16272365
-
Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia.Exp Hematol. 2010 Sep;38(9):756-64, 764.e1-4. doi: 10.1016/j.exphem.2010.05.003. Epub 2010 May 19. Exp Hematol. 2010. PMID: 20488224
-
CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2.Blood. 2004 Mar 1;103(5):1595-601. doi: 10.1182/blood-2003-05-1643. Epub 2003 Oct 30. Blood. 2004. PMID: 14592837
-
A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system.Cytokine Growth Factor Rev. 2013 Jun;24(3):269-83. doi: 10.1016/j.cytogfr.2013.03.001. Epub 2013 Apr 12. Cytokine Growth Factor Rev. 2013. PMID: 23587803 Review.
-
The multiple personalities of the chemokine receptor CCR7 in dendritic cells.J Immunol. 2006 May 1;176(9):5153-9. doi: 10.4049/jimmunol.176.9.5153. J Immunol. 2006. PMID: 16621978 Review.
Cited by
-
TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis.Oncogene. 2016 Feb 11;35(6):748-60. doi: 10.1038/onc.2015.133. Epub 2015 May 11. Oncogene. 2016. PMID: 25961925 Free PMC article.
-
Celiac Disease Increases the Risk of Multiple Sclerosis: Evidence from Mendelian Randomization and the Role of CCL19.Exp Neurobiol. 2025 Apr 30;34(2):63-76. doi: 10.5607/en25009. Epub 2025 Apr 15. Exp Neurobiol. 2025. PMID: 40229195 Free PMC article.
-
Gliadin Induces Neutrophil Migration via Engagement of the Formyl Peptide Receptor, FPR1.PLoS One. 2015 Sep 17;10(9):e0138338. doi: 10.1371/journal.pone.0138338. eCollection 2015. PLoS One. 2015. PMID: 26378785 Free PMC article.
-
Matrix expansion and syncytial aggregation of syndecan-1+ cells underpin villous atrophy in coeliac disease.PLoS One. 2014 Sep 8;9(9):e106005. doi: 10.1371/journal.pone.0106005. eCollection 2014. PLoS One. 2014. PMID: 25198673 Free PMC article.
-
Pepsin digest of wheat gliadin fraction increases production of IL-1β via TLR4/MyD88/TRIF/MAPK/NF-κB signaling pathway and an NLRP3 inflammasome activation.PLoS One. 2013 Apr 29;8(4):e62426. doi: 10.1371/journal.pone.0062426. Print 2013. PLoS One. 2013. PMID: 23658628 Free PMC article.
References
-
- Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–93. - PubMed
-
- Spurkland A, Sollid LM, Polanco I, et al. HLA-DR and -DQ genotypes of celiac disease patients serologically typed to be non-DR3 or non-DR5/7. Hum Immunol. 1992;35:188–92. - PubMed
-
- Molberg O, McAdam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–7. - PubMed
-
- Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

