Gliadin fragments promote migration of dendritic cells
- PMID: 20406323
- PMCID: PMC3922678
- DOI: 10.1111/j.1582-4934.2010.01066.x
Gliadin fragments promote migration of dendritic cells
Abstract
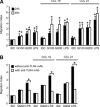
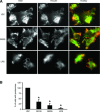
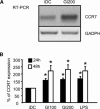
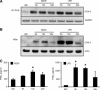
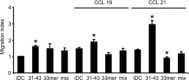
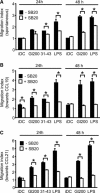
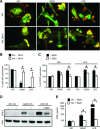
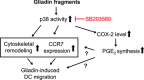
In genetically predisposed individuals, ingestion of wheat gliadin provokes a T-cell-mediated enteropathy, celiac disease. Gliadin fragments were previously reported to induce phenotypic maturation and Th1 cytokine production by human dendritic cells (DCs) and to boost their capacity to stimulate allogeneic T cells. Here, we monitor the effects of gliadin on migratory capacities of DCs. Using transwell assays, we show that gliadin peptic digest stimulates migration of human DCs and their chemotactic responsiveness to the lymph node-homing chemokines CCL19 and CCL21. The gliadin-induced migration is accompanied by extensive alterations of the cytoskeletal organization, with dissolution of adhesion structures, podosomes, as well as up-regulation of the CC chemokine receptor (CCR) 7 on cell surface and induction of cyclooxygenase (COX)-2 enzyme that mediates prostaglandin E2 (PGE₂) production. Blocking experiments confirmed that gliadin-induced migration is independent of the TLR4 signalling. Moreover, we showed that the α-gliadin-derived 31-43 peptide is an active migration-inducing component of the digest. The migration promoted by gliadin fragments or the 31-43 peptide required activation of p38 mitogen-activated protein kinase (MAPK). As revealed using p38 MAPK inhibitor SB203580, this was responsible for DC cytoskeletal transition, CCR7 up-regulation and PGE₂ production in particular. Taken together, this study provides a new insight into pathogenic features of gliadin fragments by demonstrating their ability to promote DC migration, which is a prerequisite for efficient priming of naive T cells, contributing to celiac disease pathology.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
References
-
- Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–93. - PubMed
-
- Spurkland A, Sollid LM, Polanco I, et al. HLA-DR and -DQ genotypes of celiac disease patients serologically typed to be non-DR3 or non-DR5/7. Hum Immunol. 1992;35:188–92. - PubMed
-
- Molberg O, McAdam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–7. - PubMed
-
- Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

