Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model
- PMID: 20406327
- PMCID: PMC2998585
- DOI: 10.1111/j.1582-4934.2010.01068.x
Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model
Abstract
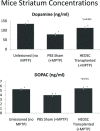
Parkinson's disease (PD) is a neurodegenerative disorder caused by the loss of dopaminergic neurons. Adult human endometrial derived stem cells (HEDSC), a readily obtainable type of mesenchymal stem-like cell, were used to generate dopaminergic cells and for transplantation. Cells expressing CD90, platelet derived growth factor (PDGF)-Rβ and CD146 but not CD45 or CD31 were differentiated in vitro into dopaminergic neurons that exhibited axon projections, pyramidal cell bodies and dendritic projections that recapitulate synapse formation; these cells also expressed the neural marker nestin and tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis. Whole cell patch clamp recording identified G-protein coupled inwardly rectifying potassium current 2 channels characteristic of central neurons. A 1-methyl 4-phenyl 1,2,3,6-tetrahydro pyridine induced animal model of PD was used to demonstrate the ability of labelled HEDSC to engraft, migrate to the site of lesion, differentiate in vivo and significantly increase striatal dopamine and dopamine metabolite concentrations. HEDSC are a highly inducible source of allogenic stem cells that rescue dopamine concentrations in an immunocompetent PD mouse model.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




Similar articles
-
Endometrial stem cell transplantation in MPTP- exposed primates: an alternative cell source for treatment of Parkinson's disease.J Cell Mol Med. 2015 Jan;19(1):249-56. doi: 10.1111/jcmm.12433. Epub 2014 Oct 6. J Cell Mol Med. 2015. PMID: 25283241 Free PMC article.
-
Dopaminergic-like neurons derived from oral mucosa stem cells by developmental cues improve symptoms in the hemi-parkinsonian rat model.PLoS One. 2014 Jun 19;9(6):e100445. doi: 10.1371/journal.pone.0100445. eCollection 2014. PLoS One. 2014. PMID: 24945922 Free PMC article.
-
Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease.Nature. 2002 Jul 4;418(6893):50-6. doi: 10.1038/nature00900. Epub 2002 Jun 20. Nature. 2002. PMID: 12077607
-
Stem cells in the treatment of Parkinson's disease.Brain Res Bull. 2002 Apr;57(6):795-808. doi: 10.1016/s0361-9230(01)00772-9. Brain Res Bull. 2002. PMID: 12031276 Review.
-
Embryonic and adult stem cells as a source for cell therapy in Parkinson's disease.J Mol Neurosci. 2004;24(3):353-86. doi: 10.1385/JMN:24:3:353. J Mol Neurosci. 2004. PMID: 15655260 Review.
Cited by
-
Human endometrial stem cells as a new source for programming to neural cells.Cell Biol Int Rep (2010). 2012 Apr 26;19(1):e00015. doi: 10.1042/CBR20110009. Cell Biol Int Rep (2010). 2012. PMID: 23124318 Free PMC article.
-
Isolation and characterization mesenchymal stem cells from red panda (Ailurus fulgens styani) endometrium.Conserv Physiol. 2022 Feb 21;10(1):coac004. doi: 10.1093/conphys/coac004. eCollection 2022 Jan 1. Conserv Physiol. 2022. PMID: 35211318 Free PMC article.
-
Evaluation of Motor Neuron-Like Cell Differentiation of hEnSCs on Biodegradable PLGA Nanofiber Scaffolds.Mol Neurobiol. 2015 Dec;52(3):1704-1713. doi: 10.1007/s12035-014-8931-2. Epub 2014 Nov 7. Mol Neurobiol. 2015. PMID: 25377792
-
Inhibitor of PI3K/Akt Signaling Pathway Small Molecule Promotes Motor Neuron Differentiation of Human Endometrial Stem Cells Cultured on Electrospun Biocomposite Polycaprolactone/Collagen Scaffolds.Mol Neurobiol. 2017 May;54(4):2547-2554. doi: 10.1007/s12035-016-9828-z. Epub 2016 Mar 18. Mol Neurobiol. 2017. PMID: 26993294
-
Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure--a pilot study.PLoS One. 2013 Dec 10;8(12):e82582. doi: 10.1371/journal.pone.0082582. eCollection 2013. PLoS One. 2013. PMID: 24340046 Free PMC article. Clinical Trial.
References
-
- Freed C, Greene P, Breeze R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–9. - PubMed
-
- Olanow C, Goetz C, Kordower J, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–14. - PubMed
-
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. - PubMed
-
- Li Y, Chen J, Wang L, et al. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci Lett. 2001;316:67–70. - PubMed
-
- Hellmann M, Panet H, Barhum Y, et al. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett. 2006;395:124–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

