Differential subcellular distributions and trafficking functions of hnRNP A2/B1 spliceoforms
- PMID: 20406423
- PMCID: PMC2906249
- DOI: 10.1111/j.1600-0854.2010.01072.x
Differential subcellular distributions and trafficking functions of hnRNP A2/B1 spliceoforms
Abstract
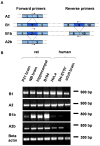
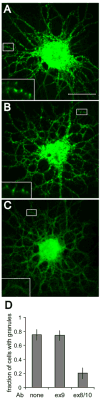
Trafficking of mRNA molecules from the nucleus to distal processes in neural cells is mediated by heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1 trans-acting factors. Although hnRNP A2/B1 is alternatively spliced to generate four isoforms, most functional studies have not distinguished between these isoforms. Here, we show, using isoform-specific antibodies and isoform-specific green fluorescent protein (GFP)-fusion expression constructs, that A2b is the predominant cytoplasmic isoform in neural cells, suggesting that it may play a key role in mRNA trafficking. The differential subcellular distribution patterns of the individual isoforms are determined by the presence or absence of alternative exons that also affect their dynamic behavior in different cellular compartments, as measured by fluorescence correlation spectroscopy. Expression of A2b is also differentially regulated with age, species and cellular development. Furthermore, coinjection of isoform-specific antibodies and labeled RNA into live oligodendrocytes shows that the assembly of RNA granules is impaired by blockade of A2b function. These findings suggest that neural cells modulate mRNA trafficking by regulating alternative splicing of hnRNP A2/B1 and controlling expression levels of A2b, which may be the predominant mediator of cytoplasmic-trafficking functions. These findings highlight the importance of considering isoform-specific functions for alternatively spliced proteins.
Figures









References
-
- Sossin WS, DesGroseillers L. Intracellular trafficking of RNA in neurons. Traffic. 2006;7(12):1581–1589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

