Pelota interacts with HAX1, EIF3G and SRPX and the resulting protein complexes are associated with the actin cytoskeleton
- PMID: 20406461
- PMCID: PMC2867792
- DOI: 10.1186/1471-2121-11-28
Pelota interacts with HAX1, EIF3G and SRPX and the resulting protein complexes are associated with the actin cytoskeleton
Abstract
Background: Pelota (PELO) is an evolutionary conserved protein, which has been reported to be involved in the regulation of cell proliferation and stem cell self-renewal. Recent studies revealed the essential role of PELO in the No-Go mRNA decay, by which mRNA with translational stall are endonucleotically cleaved and degraded. Further, PELO-deficient mice die early during gastrulation due to defects in cell proliferation and/or differentiation.
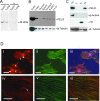
Results: We show here that PELO is associated with actin microfilaments of mammalian cells. Overexpression of human PELO in Hep2G cells had prominent effect on cell growth, cytoskeleton organization and cell spreading. To find proteins interacting with PELO, full-length human PELO cDNA was used as a bait in a yeast two-hybrid screening assay. Partial sequences of HAX1, EIF3G and SRPX protein were identified as PELO-interacting partners from the screening. The interactions between PELO and HAX1, EIF3G and SRPX were confirmed in vitro by GST pull-down assays and in vivo by co-immunoprecipitation. Furthermore, the PELO interaction domain was mapped to residues 268-385 containing the c-terminal and acidic tail domain. By bimolecular fluorescence complementation assay (BiFC), we found that protein complexes resulting from the interactions between PELO and either HAX1, EIF3G or SRPX were mainly localized to cytoskeletal filaments.
Conclusion: We could show that PELO is subcellularly localized at the actin cytoskeleton, interacts with HAX1, EIF3G and SRPX proteins and that this interaction occurs at the cytoskeleton. Binding of PELO to cytoskeleton-associated proteins may facilitate PELO to detect and degrade aberrant mRNAs, at which the ribosome is stalled during translation.
Figures




Similar articles
-
Apoptosis-inducing factor (AIF) inhibits protein synthesis by interacting with the eukaryotic translation initiation factor 3 subunit p44 (eIF3g).FEBS Lett. 2006 Nov 27;580(27):6375-83. doi: 10.1016/j.febslet.2006.10.049. Epub 2006 Nov 3. FEBS Lett. 2006. PMID: 17094969
-
The interactome of multifunctional HAX1 protein suggests its role in the regulation of energy metabolism, de-aggregation, cytoskeleton organization and RNA-processing.Biosci Rep. 2020 Nov 27;40(11):BSR20203094. doi: 10.1042/BSR20203094. Biosci Rep. 2020. PMID: 33146709 Free PMC article.
-
hSav1 interacts with HAX1 and attenuates its anti-apoptotic effects in MCF-7 breast cancer cells.Int J Mol Med. 2011 Sep;28(3):349-55. doi: 10.3892/ijmm.2011.692. Epub 2011 May 6. Int J Mol Med. 2011. PMID: 21567072
-
Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton.Trends Cell Biol. 2007 Sep;17(9):428-37. doi: 10.1016/j.tcb.2007.06.006. Epub 2007 Sep 4. Trends Cell Biol. 2007. PMID: 17804239 Review.
-
Actin Post-translational Modifications: The Cinderella of Cytoskeletal Control.Trends Biochem Sci. 2019 Jun;44(6):502-516. doi: 10.1016/j.tibs.2018.11.010. Epub 2019 Jan 2. Trends Biochem Sci. 2019. PMID: 30611609 Review.
Cited by
-
eIF3: a factor for human health and disease.RNA Biol. 2018 Jan 2;15(1):26-34. doi: 10.1080/15476286.2017.1391437. Epub 2017 Nov 13. RNA Biol. 2018. PMID: 29099306 Free PMC article. Review.
-
Translation initiation factors eIF3 and HCR1 control translation termination and stop codon read-through in yeast cells.PLoS Genet. 2013 Nov;9(11):e1003962. doi: 10.1371/journal.pgen.1003962. Epub 2013 Nov 21. PLoS Genet. 2013. PMID: 24278036 Free PMC article.
-
Loss of human disease protein retinitis pigmentosa GTPase regulator (RPGR) differentially affects rod or cone-enriched retina.Hum Mol Genet. 2016 Apr 1;25(7):1345-56. doi: 10.1093/hmg/ddw017. Epub 2016 Jan 24. Hum Mol Genet. 2016. PMID: 26908598 Free PMC article.
-
Surveillance pathways rescuing eukaryotic ribosomes lost in translation.Nat Rev Mol Cell Biol. 2012 Nov;13(11):727-35. doi: 10.1038/nrm3457. Epub 2012 Oct 17. Nat Rev Mol Cell Biol. 2012. PMID: 23072885 Review.
-
Nuclear distribution of eIF3g and its interacting nuclear proteins in breast cancer cells.Mol Med Rep. 2016 Apr;13(4):2973-80. doi: 10.3892/mmr.2016.4935. Epub 2016 Feb 23. Mol Med Rep. 2016. PMID: 26935993 Free PMC article.
References
-
- Eberhart CG, Wasserman SA. The pelota locus encodes a protein required for meiotic cell division: an analysis of G2/M arrest in Drosophila spermatogenesis. Development. 1995;121:3477–3486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

