CEACAM1 recognition by bacterial pathogens is species-specific
- PMID: 20406467
- PMCID: PMC2871271
- DOI: 10.1186/1471-2180-10-117
CEACAM1 recognition by bacterial pathogens is species-specific
Abstract
Background: Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), an immunoglobulin (Ig)-related glycoprotein, serves as cellular receptor for a variety of Gram-negative bacterial pathogens associated with the human mucosa. In particular, Neisseria gonorrhoeae, N. meningitidis, Moraxella catarrhalis, and Haemophilus influenzae possess well-characterized CEACAM1-binding adhesins. CEACAM1 is typically involved in cell-cell attachment, epithelial differentiation, neovascularisation and regulation of T-cell proliferation, and is one of the few CEACAM family members with homologues in different mammalian lineages. However, it is unknown whether bacterial adhesins of human pathogens can recognize CEACAM1 orthologues from other mammals.
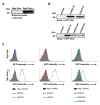
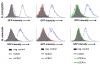
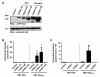
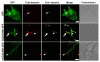
Results: Sequence comparisons of the amino-terminal Ig-variable-like domain of CEACAM1 reveal that the highest sequence divergence between human, murine, canine and bovine orthologues is found in the beta-strands comprising the bacteria-binding CC'FG-face of the Ig-fold. Using GFP-tagged, soluble amino-terminal domains of CEACAM1, we demonstrate that bacterial pathogens selectively associate with human, but not other mammalian CEACAM1 orthologues. Whereas full-length human CEACAM1 can mediate internalization of Neisseria gonorrhoeae in transfected cells, murine CEACAM1 fails to support bacterial internalization, demonstrating that the sequence divergence of CEACAM1 orthologues has functional consequences with regard to bacterial recognition and cellular invasion.
Conclusions: Our results establish the selective interaction of several human-restricted bacterial pathogens with human CEACAM1 and suggest that co-evolution of microbial adhesins with their corresponding receptors on mammalian cells contributes to the limited host range of these highly adapted infectious agents.
Figures

Similar articles
-
Neisseria meningitidis has two independent modes of recognizing its human receptor CEACAM1.PLoS One. 2011 Jan 27;6(1):e14609. doi: 10.1371/journal.pone.0014609. PLoS One. 2011. PMID: 21298042 Free PMC article.
-
Extracellular IgC2 constant domains of CEACAMs mediate PI3K sensitivity during uptake of pathogens.PLoS One. 2012;7(6):e39908. doi: 10.1371/journal.pone.0039908. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768164 Free PMC article.
-
Specific Binding to Differentially Expressed Human Carcinoembryonic Antigen-Related Cell Adhesion Molecules Determines the Outcome of Neisseria gonorrhoeae Infections along the Female Reproductive Tract.Infect Immun. 2018 Jul 23;86(8):e00092-18. doi: 10.1128/IAI.00092-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29760215 Free PMC article.
-
Temporal upregulation of host surface receptors provides a window of opportunity for bacterial adhesion and disease.Microbiology (Reading). 2017 Apr;163(4):421-430. doi: 10.1099/mic.0.000434. Epub 2017 Apr 6. Microbiology (Reading). 2017. PMID: 28113047 Review.
-
[CEACAM1 as a central modulator of metabolism, tumor progression, angiogenesis and immunity].Med Sci (Paris). 2009 Mar;25(3):247-52. doi: 10.1051/medsci/2009253247. Med Sci (Paris). 2009. PMID: 19361387 Review. French.
Cited by
-
Innate recognition by neutrophil granulocytes differs between Neisseria gonorrhoeae strains causing local or disseminating infections.Infect Immun. 2013 Jul;81(7):2358-70. doi: 10.1128/IAI.00128-13. Epub 2013 Apr 29. Infect Immun. 2013. PMID: 23630956 Free PMC article.
-
Natural selection supports escape from concerted evolution of a recently duplicated CEACAM1 paralog in the ruminant CEA gene family.Sci Rep. 2020 Feb 25;10(1):3404. doi: 10.1038/s41598-020-60425-4. Sci Rep. 2020. PMID: 32099040 Free PMC article.
-
Human CEACAM1 is targeted by a Streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis.Nat Commun. 2023 Apr 20;14(1):2275. doi: 10.1038/s41467-023-37732-1. Nat Commun. 2023. PMID: 37080973 Free PMC article.
-
CEACAM1 structure and function in immunity and its therapeutic implications.Semin Immunol. 2019 Apr;42:101296. doi: 10.1016/j.smim.2019.101296. Semin Immunol. 2019. PMID: 31604530 Free PMC article. Review.
-
Phosphatidylinositol 3'-kinase activity is critical for initiating the oxidative burst and bacterial destruction during CEACAM3-mediated phagocytosis.J Biol Chem. 2011 Mar 18;286(11):9555-66. doi: 10.1074/jbc.M110.216085. Epub 2011 Jan 7. J Biol Chem. 2011. PMID: 21216968 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

