Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections
- PMID: 20406471
- PMCID: PMC2869333
- DOI: 10.1186/1471-2121-11-29
Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections
Abstract
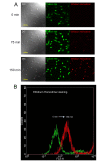
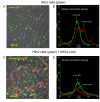
Background: Bone marrow derived mesenchymal stem cells (MSCs) are promising candidates for cell based therapies in myocardial infarction. However, the exact underlying cellular mechanisms are still not fully understood. Our aim was to explore the possible role of direct cell-to-cell interaction between ischemic H9c2 cardiomyoblasts and normal MSCs. Using an in vitro ischemia model of 150 minutes of oxygen glucose deprivation we investigated cell viability and cell interactions with confocal microscopy and flow cytometry.
Results: Our model revealed that adding normal MSCs to the ischemic cell population significantly decreased the ratio of dead H9c2 cells (H9c2 only: 0.85 +/- 0.086 vs. H9c2+MSCs: 0.16 +/- 0.035). This effect was dependent on direct cell-to-cell contact since co-cultivation with MSCs cultured in cell inserts did not exert the same beneficial effect (ratio of dead H9c2 cells: 0.90 +/- 0.055). Confocal microscopy revealed that cardiomyoblasts and MSCs frequently formed 200-500 nm wide intercellular connections and cell fusion rarely occurred between these cells.
Conclusion: Based on these results we hypothesize that mesenchymal stem cells may reduce the number of dead cardiomyoblasts after ischemic damage via direct cell-to-cell interactions and intercellular tubular connections may play an important role in these processes.
Figures




Similar articles
-
Stem cell transplantation in an in vitro simulated ischemia/reperfusion model.J Vis Exp. 2011 Nov 5;(57):e3575. doi: 10.3791/3575. J Vis Exp. 2011. PMID: 22083407 Free PMC article.
-
MiR-29a in mesenchymal stem cells inhibits FSTL1 secretion and promotes cardiac myocyte apoptosis in hypoxia-reoxygenation injury.Cardiovasc Pathol. 2020 May-Jun;46:107180. doi: 10.1016/j.carpath.2019.107180. Epub 2019 Nov 19. Cardiovasc Pathol. 2020. PMID: 31945680
-
Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model.Mol Med Rep. 2016 Feb;13(2):1517-24. doi: 10.3892/mmr.2015.4726. Epub 2015 Dec 28. Mol Med Rep. 2016. PMID: 26718099 Free PMC article.
-
MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction.Eur Rev Med Pharmacol Sci. 2020 Oct;24(19):10107-10117. doi: 10.26355/eurrev_202010_23230. Eur Rev Med Pharmacol Sci. 2020. PMID: 33090418
-
C-Met-Activated Mesenchymal Stem Cells Rescue Ischemic Damage via Interaction with Cellular Prion Protein.Cell Physiol Biochem. 2018;46(5):1835-1848. doi: 10.1159/000489368. Epub 2018 Apr 25. Cell Physiol Biochem. 2018. PMID: 29705776
Cited by
-
Transcriptional profiling of interleukin-2-primed human adipose derived mesenchymal stem cells revealed dramatic changes in stem cells response imposed by replicative senescence.Oncotarget. 2015 Jul 20;6(20):17938-57. doi: 10.18632/oncotarget.4852. Oncotarget. 2015. PMID: 26255627 Free PMC article.
-
Are the Properties of Bone Marrow-Derived Mesenchymal Stem Cells Influenced by Overweight and Obesity?Int J Mol Sci. 2023 Mar 2;24(5):4831. doi: 10.3390/ijms24054831. Int J Mol Sci. 2023. PMID: 36902259 Free PMC article. Review.
-
Regenerative abilities of mesenchymal stem cells through mitochondrial transfer.J Biomed Sci. 2018 Mar 30;25(1):31. doi: 10.1186/s12929-018-0429-1. J Biomed Sci. 2018. PMID: 29602309 Free PMC article. Review.
-
CIRBP protects H9C2 cells against myocardial ischemia through inhibition of NF-κB pathway.Braz J Med Biol Res. 2017 Mar 23;50(4):e5861. doi: 10.1590/1414-431X20175861. Braz J Med Biol Res. 2017. PMID: 28355355 Free PMC article.
-
Mitochondrial transplantation as a novel therapeutic strategy for cardiovascular diseases.J Transl Med. 2023 May 25;21(1):347. doi: 10.1186/s12967-023-04203-6. J Transl Med. 2023. PMID: 37231493 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

