High-efficiency transfection of cultured primary motor neurons to study protein localization, trafficking, and function
- PMID: 20406490
- PMCID: PMC2867961
- DOI: 10.1186/1750-1326-5-17
High-efficiency transfection of cultured primary motor neurons to study protein localization, trafficking, and function
Abstract
Background: Cultured spinal motor neurons are a valuable tool to study basic mechanisms of development, axon growth and pathfinding, and, importantly, to analyze the pathomechanisms underlying motor neuron diseases. However, the application of this cell culture model is limited by the lack of efficient gene transfer techniques which are available for other neurons. To address this problem, we have established magnetofection as a novel method for the simple and efficient transfection of mouse embryonic motor neurons. This technique allows for the study of the effects of gene expression and silencing on the development and survival of motor neurons.
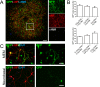
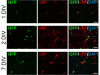
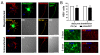
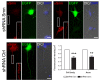
Results: We found that magnetofection, a novel transfection technology based on the delivery of DNA-coated magnetic nanobeads, can be used to transfect primary motor neurons. Therefore, in order to use this method as a new tool for studying the localization and transport of axonal proteins, we optimized conditions and determined parameters for efficient transfection rates of >45% while minimizing toxic effects on survival and morphology. To demonstrate the potential of this method, we have used transfection with plasmids encoding fluorescent fusion-proteins to show for the first time that the spinal muscular atrophy-disease protein Smn is actively transported along axons of live primary motor neurons, supporting an axon-specific role for Smn that is different from its canonical function in mRNA splicing. We were also able to show the suitability of magnetofection for gene knockdown with shRNA-based constructs by significantly reducing Smn levels in both cell bodies and axons, opening new opportunities for the study of the function of axonal proteins in motor neurons.
Conclusions: In this study we have established an optimized magnetofection protocol as a novel transfection method for primary motor neurons that is simple, efficient and non-toxic. We anticipate that this novel approach will have a broad applicability in the study of motor neuron development, axonal trafficking, and molecular mechanisms of motor neuron diseases.
Figures


References
-
- Cisterni C, Henderson CE, Aebischer P, Pettmann B, Deglon N. Efficient gene transfer and expression of biologically active glial cell line-derived neurotrophic factor in rat motoneurons transduced wit lentiviral vectors. J Neurochem. 2000;74:1820–1828. doi: 10.1046/j.1471-4159.2000.0741820.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

