Development of a repressible mycobacterial promoter system based on two transcriptional repressors
- PMID: 20406773
- PMCID: PMC2896539
- DOI: 10.1093/nar/gkq235
Development of a repressible mycobacterial promoter system based on two transcriptional repressors
Abstract
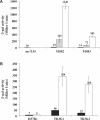
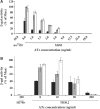
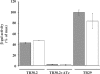
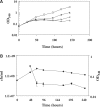
Tightly regulated gene expression systems represent invaluable tools for studying gene function and for the validation of drug targets in bacteria. While several regulated bacterial promoters have been characterized, few of them have been successfully used in mycobacteria. In this article we describe the development of a novel repressible promoter system effective in both fast- and slow-growing mycobacteria based on two chromosomally encoded repressors, dependent on tetracycline (TetR) and pristinamycin (Pip), respectively. This uniqueness results in high versatility and stringency. Using this method we were able to obtain an ftsZ conditional mutant in Mycobacterium smegmatis and a fadD32 conditional mutant in Mycobacterium tuberculosis, confirming their essentiality for bacterial growth in vitro. This repressible promoter system could also be exploited to regulate gene expression during M. tuberculosis intracellular growth.
Figures


References
-
- Vitoria M, Granich R, Gilks CF, Gunneberg C, Hosseini M, Were W, Raviglione M, De Cock KM. The global fight against HIV/AIDS, tuberculosis, and malaria: current status and future perspectives. Am. J. Clin. Pathol. 2009;131:844–848. - PubMed
-
- Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, Hoffner S, Drobniewski F, Barrera L, van Soolingen D, et al. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–1873. - PubMed
-
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. - PubMed
-
- Parish T, Mahenthiralingam E, Draper P, Davis EO, Colston MJ. Regulation of the inducible acetamidase gene of Mycobacterium smegmatis. Microbiology. 1997;143(Pt 7):2267–2276. - PubMed
-
- Triccas JA, Parish T, Britton WJ, Gicquel B. An inducible expression system permitting the efficient purification of a recombinant antigen from Mycobacterium smegmatis. FEMS Microbiol. Lett. 1998;167:151–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

