Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells
- PMID: 20406815
- PMCID: PMC2888439
- DOI: 10.1074/jbc.M110.132662
Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells
Abstract
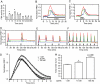
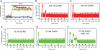
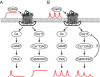
The hypothalamic neuropeptide gonadotropin-releasing hormone (GnRH) is secreted in a pulsatile fashion by hypothalamic neurons, and alterations in pulse frequency and amplitude differentially regulate gonadotropin synthesis and release. In this study, we investigated the kinetics of G(s) and G(q) signaling in response to continuous or pulsatile GnRH using fluorescence resonance energy transfer reporters in live mouse LbetaT2 gonadotrope cells. cAMP and protein kinase A-dependent reporters showed a rapid but transient increase in fluorescence resonance energy transfer signal with increasing doses of constant GnRH, and in contrast diacylglycerol (DAG) and calcium reporters showed a rapid and sustained signal. Multiple pulses of GnRH caused multiple pulses of cAMP and protein kinase A activation without desensitization, but the DAG and calcium reporters were rapidly desensitized resulting in inhibition of calcium and DAG responses. At the transcriptional level, both a cAMP-dependent cAMP-response element reporter and a DAG/calcium-dependent AP-1 reporter showed a pulse frequency-dependent increase in luciferase activity. However, constant GnRH stimulation gave very little cAMP-response element activation but very strong AP-1 activation. Based on these data, we propose that both the GnRH-R-G(s) and G(q) pathways are responsive to pulses of GnRH, but only the G(q) pathway is responsive to constant GnRH. Furthermore, the G(q) pathway is subject to desensitization with multiple GnRH pulses, but the G(s) pathway is not.
Figures




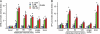
References
-
- Conn P. M., Crowley W. F., Jr. (1994) Annu. Rev. Med. 45, 391–405 - PubMed
-
- Kaiser U. B., Conn P. M., Chin W. W. (1997) Endocr. Rev. 18, 46–70 - PubMed
-
- Marshall J. C., Eagleson C. A., McCartney C. R. (2001) Mol. Cell. Endocrinol. 183, 29–32 - PubMed
-
- Grosse R., Schmid A., Schöneberg T., Herrlich A., Muhn P., Schultz G., Gudermann T. (2000) J. Biol. Chem. 275, 9193–9200 - PubMed
-
- Han X. B., Conn P. M. (1999) Endocrinology 140, 2241–2251 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

