Identification of Drosophila Yin and PEPT2 as evolutionarily conserved phagosome-associated muramyl dipeptide transporters
- PMID: 20406817
- PMCID: PMC2888427
- DOI: 10.1074/jbc.M110.115584
Identification of Drosophila Yin and PEPT2 as evolutionarily conserved phagosome-associated muramyl dipeptide transporters
Abstract
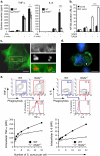
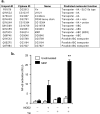
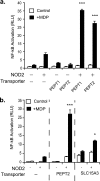
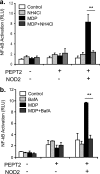
NOD2 (nucleotide-binding oligomerization domain containing 2) is an important cytosolic pattern recognition receptor that activates NF-kappaB and other immune effector pathways such as autophagy and antigen presentation. Despite its intracellular localization, NOD2 participates in sensing of extracellular microbes such as Staphylococcus aureus. NOD2 ligands similar to the minimal synthetic ligand muramyl dipeptide (MDP) are generated by internalization and processing of bacteria in hydrolytic phagolysosomes. However, how these derived ligands exit this organelle and access the cytosol to activate NOD2 is poorly understood. Here, we address how phagosome-derived NOD2 ligands access the cytosol in human phagocytes. Drawing on data from Drosophila phagosomes, we identify an evolutionarily conserved role of SLC15A transporters, Drosophila Yin and PEPT2, as MDP transporters in fly and human phagocytes, respectively. We show that PEPT2 is highly expressed by human myeloid cells. Ectopic expression of both Yin and PEPT2 increases the sensitivity of NOD2-dependent NF-kappaB activation. Additionally, we show that PEPT2 associates with phagosome membranes. Together, these data identify Drosophila Yin and PEPT2 as evolutionarily conserved phagosome-associated transporters that are likely to be of particular importance in delivery of bacteria-derived ligands generated in phagosomes to cytosolic sensors recruited to the vicinity of these organelles.
Figures

Similar articles
-
Endosomes are specialized platforms for bacterial sensing and NOD2 signalling.Nature. 2014 May 8;509(7499):240-4. doi: 10.1038/nature13133. Epub 2014 Mar 30. Nature. 2014. PMID: 24695226
-
Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation.J Immunol. 2009 Apr 1;182(7):4321-7. doi: 10.4049/jimmunol.0802197. J Immunol. 2009. PMID: 19299732 Free PMC article.
-
Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils.Immunology. 2014 Oct;143(2):269-76. doi: 10.1111/imm.12307. Immunology. 2014. PMID: 24766550 Free PMC article.
-
Muramyl dipeptide responsive pathways in Crohn's disease: from NOD2 and beyond.Cell Mol Life Sci. 2013 Sep;70(18):3391-404. doi: 10.1007/s00018-012-1246-4. Epub 2012 Dec 29. Cell Mol Life Sci. 2013. PMID: 23275943 Free PMC article. Review.
-
Structure-activity relationship in NOD2 agonistic muramyl dipeptides.Eur J Med Chem. 2024 May 5;271:116439. doi: 10.1016/j.ejmech.2024.116439. Epub 2024 Apr 20. Eur J Med Chem. 2024. PMID: 38691886 Free PMC article. Review.
Cited by
-
Attenuating ABHD17 Isoforms Augments the S-acylation and Function of NOD2 and a Subset of Crohn's Disease-associated NOD2 Variants.Cell Mol Gastroenterol Hepatol. 2025;19(6):101491. doi: 10.1016/j.jcmgh.2025.101491. Epub 2025 Mar 5. Cell Mol Gastroenterol Hepatol. 2025. PMID: 40054525 Free PMC article.
-
Function, Regulation, and Pathophysiological Relevance of the POT Superfamily, Specifically PepT1 in Inflammatory Bowel Disease.Compr Physiol. 2018 Mar 25;8(2):731-760. doi: 10.1002/cphy.c170032. Compr Physiol. 2018. PMID: 29687900 Free PMC article. Review.
-
Membrane Association Dictates Ligand Specificity for the Innate Immune Receptor NOD2.ACS Chem Biol. 2017 Aug 18;12(8):2216-2224. doi: 10.1021/acschembio.7b00469. Epub 2017 Jul 25. ACS Chem Biol. 2017. PMID: 28708377 Free PMC article.
-
Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster.Genetics. 2018 Oct;210(2):357-396. doi: 10.1534/genetics.118.300224. Genetics. 2018. PMID: 30287514 Free PMC article. Review.
-
Microbiome composition shapes temperature tolerance in a Hawaiian picture-winged Drosophila.bioRxiv [Preprint]. 2025 Jun 7:2025.06.03.657679. doi: 10.1101/2025.06.03.657679. bioRxiv. 2025. Update in: J Exp Biol. 2025 Jul 22:jeb.250973. doi: 10.1242/jeb.250973. PMID: 40501599 Free PMC article. Updated. Preprint.
References
-
- Hoffmann J. A., Kafatos F. C., Janeway C. A., Ezekowitz R. A. (1999) Science 284, 1313–1318 - PubMed
-
- Janeway C. A., Jr., Medzhitov R. (2002) Annu. Rev. Immunol. 20, 197–216 - PubMed
-
- Takeda K., Kaisho T., Akira S. (2003) Annu. Rev. Immunol. 21, 335–376 - PubMed
-
- Inohara N., Nuñez G. (2003) Nat. Rev. Immunol. 3, 371–382 - PubMed
-
- Viala J., Sansonetti P., Philpott D. J. (2004) C. R. Biol. 327, 551–555 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

