Laser-induced propagation and destruction of amyloid beta fibrils
- PMID: 20406822
- PMCID: PMC2885244
- DOI: 10.1074/jbc.M109.076505
Laser-induced propagation and destruction of amyloid beta fibrils
Abstract
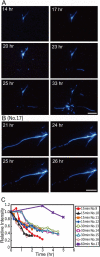
The amyloid deposition of amyloid beta (Abeta) peptides is a critical pathological event in Alzheimer disease (AD). Preventing the formation of amyloid deposits and removing preformed fibrils in tissues are important therapeutic strategies against AD. Previously, we reported the destruction of amyloid fibrils of beta(2)-microglobulin K3 fragments by laser irradiation coupled with the binding of amyloid-specific thioflavin T. Here, we studied the effects of a laser beam on Abeta fibrils. As was the case for K3 fibrils, extensive irradiation destroyed the preformed Abeta fibrils. However, irradiation during spontaneous fibril formation resulted in only the partial destruction of growing fibrils and a subsequent explosive propagation of fibrils. The explosive propagation was caused by an increase in the number of active ends due to breakage. The results not only reveal a case of fragmentation-induced propagation of fibrils but also provide insights into therapeutic strategies for AD.
Figures



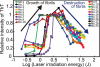
Similar articles
-
Destruction of amyloid fibrils of keratoepithelin peptides by laser irradiation coupled with amyloid-specific thioflavin T.J Biol Chem. 2011 Mar 25;286(12):10856-63. doi: 10.1074/jbc.M111.222901. Epub 2011 Feb 7. J Biol Chem. 2011. PMID: 21300800 Free PMC article.
-
RNA aptamers generated against oligomeric Abeta40 recognize common amyloid aptatopes with low specificity but high sensitivity.PLoS One. 2009 Nov 10;4(11):e7694. doi: 10.1371/journal.pone.0007694. PLoS One. 2009. PMID: 19901993 Free PMC article.
-
S14G-humanin inhibits Aβ1-42 fibril formation, disaggregates preformed fibrils, and protects against Aβ-induced cytotoxicity in vitro.J Pept Sci. 2013 Mar;19(3):159-65. doi: 10.1002/psc.2484. Epub 2013 Jan 24. J Pept Sci. 2013. Retraction in: J Pept Sci. 2016 Jun;22(6):434. doi: 10.1002/psc.2889. PMID: 23349038 Retracted.
-
Molecular Dynamics Simulation Studies on the Aggregation of Amyloid-β Peptides and Their Disaggregation by Ultrasonic Wave and Infrared Laser Irradiation.Molecules. 2022 Apr 12;27(8):2483. doi: 10.3390/molecules27082483. Molecules. 2022. PMID: 35458686 Free PMC article. Review.
-
Structural polymorphism of Alzheimer Abeta and other amyloid fibrils.Prion. 2009 Apr-Jun;3(2):89-93. doi: 10.4161/pri.3.2.8859. Prion. 2009. PMID: 19597329 Free PMC article. Review.
Cited by
-
Alzheimer's Therapeutic Strategy: Photoactive Platforms for Suppressing the Aggregation of Amyloid β Protein.Front Chem. 2020 Jul 21;8:509. doi: 10.3389/fchem.2020.00509. eCollection 2020. Front Chem. 2020. PMID: 32793545 Free PMC article. Review.
-
Hexafluoroisopropanol induces amyloid fibrils of islet amyloid polypeptide by enhancing both hydrophobic and electrostatic interactions.J Biol Chem. 2011 Jul 8;286(27):23959-66. doi: 10.1074/jbc.M111.226688. Epub 2011 May 12. J Biol Chem. 2011. PMID: 21566116 Free PMC article.
-
Atomic Force Microscopy Analysis of EPPS-Driven Degradation and Reformation of Amyloid-β Aggregates.J Alzheimers Dis Rep. 2018 Feb 16;2(1):41-49. doi: 10.3233/ADR-170024. J Alzheimers Dis Rep. 2018. PMID: 30480247 Free PMC article.
-
Deciphering the Disaggregation Mechanism of Amyloid Beta Aggregate by 4-(2-Hydroxyethyl)-1-Piperazinepropanesulfonic Acid Using Electrochemical Impedance Spectroscopy.Sensors (Basel). 2021 Jan 25;21(3):788. doi: 10.3390/s21030788. Sensors (Basel). 2021. PMID: 33503934 Free PMC article.
-
Target-driven supramolecular self-assembly for selective amyloid-β photooxygenation against Alzheimer's disease.Chem Sci. 2020 Oct 6;11(40):11003-11008. doi: 10.1039/d0sc04984k. Chem Sci. 2020. PMID: 34094349 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

