Type I interferon receptor is a primary regulator of target-mediated drug disposition of interferon-beta in mice
- PMID: 20406858
- PMCID: PMC2912044
- DOI: 10.1124/jpet.110.167650
Type I interferon receptor is a primary regulator of target-mediated drug disposition of interferon-beta in mice
Abstract
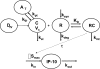
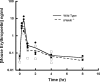
The purpose of this study is to evaluate the primary mechanism through which interferon (IFN)-beta exhibits target-mediated drug disposition (TMDD) and whether the theoretical assumptions of TMDD models are consistent with experimental pharmacokinetic (PK) data. Recombinant murine IFN-beta was administered as an intravenous injection at two dose levels (0.5 and 1 million IU/kg) to male wild-type (WT) and type-I IFN-alpha/beta receptor subunit (IFNAR-1) knockout (KO) mice (A129S7/SvEvBrd strain). Sampling was conducted at various times (n = 3/time point), and plasma was analyzed for IFN-beta concentrations using a validated enzyme-linked immunosorbent assay. The pharmacodynamic (PD) biomarker was IP-10 mRNA that was isolated from the distal femur bone and quantified using reverse transcription-polymerase chain reaction. An integrated model that includes rapid-binding TMDD and an indirect mechanism of drug action was used to characterize the PK/PD profiles. For an experimental control, PK profiles of recombinant murine erythropoietin (muEPO), another drug that exhibits TMDD, were determined after a single intravenous dose (0.5 microg/kg) in WT and KO animals. The concentration-time profiles for IFN-beta differed substantially at initial times for the WT and KO mice at the same dose levels. These differences are characteristic of ligands exhibiting receptor-mediated disposition and were well described by a rapid-binding TMDD model. No differences in muEPO PK were observed in the control study. In summary, the intact IFNAR receptor is a primary regulator of in vivo IFN-beta exposure. An integrated PK/PD model was successfully used to assess the receptor-mediated disposition and dynamics of IFN-beta.
Figures
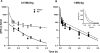

References
-
- Abraham AK, Ramanathan M, Weinstock-Guttman B, Mager DE. (2009) Mechanisms of interferon-beta effects on bone homeostasis. Biochem Pharmacol 77:1757–1762 - PubMed
-
- Agoram BM, Martin SW, van der Graaf PH. (2007) The role of mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modelling in translational research of biologics. Drug Discov Today 12:1018–1024 - PubMed
-
- Buchwalder PA, Buclin T, Trinchard I, Munafo A, Biollaz J. (2000) Pharmacokinetics and pharmacodynamics of IFN-beta 1a in healthy volunteers. J Interferon Cytokine Res 20:857–866 - PubMed
-
- Chapel S, Veng-Pedersen P, Hohl RJ, Schmidt RL, McGuire EM, Widness JA. (2001) Changes in erythropoietin pharmacokinetics following busulfan-induced bone marrow ablation in sheep: evidence for bone marrow as a major erythropoietin elimination pathway. J Pharmacol Exp Ther 298:820–824 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

