Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction
- PMID: 20406881
- PMCID: PMC3009378
- DOI: 10.1148/radiol.10091735
Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction
Abstract
Purpose: To test, in a murine model of unilateral ureteral obstruction (UUO), whether the magnetic resonance (MR) imaging-derived apparent diffusion coefficient (ADC) changes during the progression of renal fibrosis and correlates with the histopathologic changes observed in renal fibrogenesis.
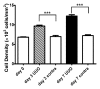
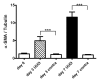
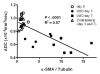
Materials and methods: This study was approved by the institutional animal care and use committee. A UUO was created in each of 14 mice. In five mice, longitudinal diffusion-weighted (DW) imaging was performed before the UUO (day 0) and on days 3 and 7 after the UUO and was followed by histopathologic analysis. The nine remaining mice were examined with cross-sectional studies on days 0 (n = 4) and 3 (n = 5). ADCs were measured with a spin-echo echo-planar sequence at five b values ranging from 350 to 1200 sec/mm(2). Differences in ADC among the time points and between the sides were assessed by using Tukey-Kramer and Student t tests, respectively. ADC was correlated with cell density and alpha-smooth muscle actin (alpha-SMA, a marker of myofibroblasts) expression at linear regression analysis.
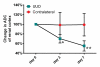
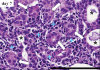
Results: Histopathologic examination revealed typical renal fibrosis on the side with UUO. The ADC decreased over time on the UUO side, from (1.02 +/- 0.06 [standard deviation]) x 10(-3) mm(2)/sec on day 0 to (0.70 +/- 0.08) x 10(-3) mm(2)/sec on day 3 (P < .001) and (0.57 +/- 0.10) x 10(-3) mm(2)/sec on day 7 (P < .001). The percentage change in ADC was greater on the UUO side than on the contralateral side on days 3 (29% +/- 9, P = .05) and 7 (44% +/- 11, P < .01). ADC correlated with both increased cell density and increased alpha-SMA expression (P < .001 for both correlations).
Conclusion: An ADC decrease in renal fibrosis is associated with an increased number of cells, including fibroblasts. ADC has the potential to serve as a sensitive noninvasive biomarker of renal fibrosis.
Copyright RSNA, 2010
Figures
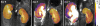




Comment in
-
Science to practice: Can diffusion-weighted MR imaging findings be used as biomarkers to monitor the progression of renal fibrosis?Radiology. 2010 Jun;255(3):667-8. doi: 10.1148/radiol.100211. Radiology. 2010. PMID: 20501704
References
-
- Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis 2009;53(3suppl 3):S4–S16 - PubMed
-
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298(17):2038–2047 - PubMed
-
- Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol 2009;296(6):F1239–F1244 - PubMed
-
- Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992;20(1):1–17 - PubMed
-
- Bohle A, Wehrmann M, Bogenschütz O, Batz C, Müller CA, Müller GA. The pathogenesis of chronic renal failure in diabetic nephropathy: investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract 1991;187(2-3):251–259 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

