MUC1 regulates nuclear localization and function of the epidermal growth factor receptor
- PMID: 20406885
- PMCID: PMC2864713
- DOI: 10.1242/jcs.062661
MUC1 regulates nuclear localization and function of the epidermal growth factor receptor
Abstract
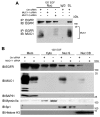
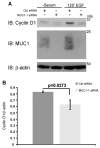
Alteration of protein trafficking and localization is associated with several diseases, including cystic fibrosis, breast cancer, colorectal cancer, leukemia and diabetes. Specifically, aberrant nuclear localization of the epidermal growth factor receptor (EGFR), a receptor tyrosine kinase, is a poor prognostic indicator in several epithelial carcinomas. It is now appreciated that in addition to signaling from the plasma membrane, EGFR also trafficks to the nucleus, and can directly bind the promoter regions of genes encoding cyclin D1 (CCND1) and B-Myb (MYBL2). We have previously established that loss of MUC1 in an EGFR-dependent transgenic mouse model of breast cancer correlates with the loss of cyclin D1 expression. Here, we provide evidence for a novel regulatory function of MUC1 in the trafficking and nuclear activity of EGFR. We found that MUC1 and EGFR interact in the nucleus of breast cancer cells, which promotes the accumulation of chromatin-bound EGFR. Additionally, the presence of MUC1 results in significant colocalization of EGFR and phosphorylated RNA polymerase II, indicating that MUC1 influences the association of EGFR with transcriptionally active promoter regions. Importantly, we found that the loss of MUC1 expression resulted in a decrease in the interaction between EGFR and the CCND1 promoter, which translated to a significant decrease in cyclin D1 protein expression. This data offers insights into a novel regulatory mechanism of EGFR nuclear function and could have important implications for evaluating nuclear localization in cancer.
Figures





References
-
- Aggarwal P., Lessie M. D., Lin D. I., Pontano L., Gladden A. B., Nuskey B., Goradia A., Wasik M. A., Klein-Szanto A. J., Rustgi A. K., et al. (2007). Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev. 21, 2908-2922 - PMC - PubMed
-
- Davis J. R., Kakar M., Lim C. S. (2007). Controlling protein compartmentalization to overcome disease. Pharm. Res. 24, 17-27 - PubMed
-
- Feng Y., Venema V. J., Venema R. C., Tsai N., Caldwell R. B. (1999). VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem. Biophys. Res. Commun. 256, 192-197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

