Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent
- PMID: 20406898
- PMCID: PMC2940627
- DOI: 10.1093/neuonc/nop048
Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent
Abstract
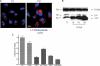
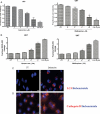
Glioblastoma (GBM) is a high-grade central nervous system malignancy and despite aggressive treatment strategies, GBM patients have a median survival time of just 1 year. Chloroquine (CQ), an antimalarial lysosomotropic agent, has been identified as a potential adjuvant in the treatment regimen of GBMs. However, the mechanism of CQ-induced tumor cell death is poorly defined. We and others have shown that CQ-mediated cell death may be p53-dependent and at least in part due to the intrinsic apoptotic death pathway. Here, we investigated the effects of CQ on 5 established human GBM lines, differing in their p53 gene status. CQ was found to induce a concentration-dependent death in each of these cell lines. Although CQ treatment increased caspase-3-like enzymatic activity in all 5 cell lines, a broad-spectrum caspase inhibitor did not significantly attenuate death. Moreover, CQ caused an accumulation of autophagic vacuoles in all cell lines and was found to affect the levels and subcellular distribution of cathepsin D, suggesting that altered lysosomal function may also play a role in CQ-induced cell death. Thus, CQ can induce p53-independent death in gliomas that do not require caspase-mediated apoptosis. To potentially identify more potent chemotherapeutics, various CQ derivatives and lysosomotropic compounds were tested on the GBM cells. Quinacrine and mefloquine were found to be more potent than CQ in killing GBM cells in vitro and given their superior blood-brain barrier penetration compared with CQ may prove more efficacious as chemotherapeutic agents for GBM patients.
Figures


References
-
- Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC. Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: analysis of 625 cases. Br J Neurosurg. 2007;21:496–500. - PubMed
-
- Utsuki S, Oka H, Suzuki S, et al. Pathological and clinical features of cystic and noncystic glioblastomas. Brain Tumor Pathol. 2006;23:29–34. - PubMed
-
- Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–343. - PubMed
-
- Briceno E, Reyes S, Sotelo J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg Focus. 2003;14 e3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

