Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering
- PMID: 20406908
- PMCID: PMC2867676
- DOI: 10.1073/pnas.1000302107
Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering
Abstract
Mesenchymal stem/stromal cells (MSC) are typically used to generate bone tissue by a process resembling intramembranous ossification, i.e., by direct osteoblastic differentiation. However, most bones develop by endochondral ossification, i.e., via remodeling of hypertrophic cartilaginous templates. To date, endochondral bone formation has not been reproduced using human, clinically compliant cell sources. Here, we aimed at engineering tissues from bone marrow-derived, adult human MSC with an intrinsic capacity to undergo endochondral ossification. By analogy to embryonic limb development, we hypothesized that successful execution of the endochondral program depends on the initial formation of hypertrophic cartilaginous templates. Human MSC, subcutaneously implanted into nude mice at various stages of chondrogenic differentiation, formed bone trabeculae only when they had developed in vitro hypertrophic tissue structures. Advanced maturation in vitro resulted in accelerated formation of larger bony tissues. The underlying morphogenetic process was structurally and molecularly similar to the temporal and spatial progression of limb bone development in embryos. In particular, Indian hedgehog signaling was activated at early stages and required for the in vitro formation of hypertrophic cartilage. Subsequent development of a bony collar in vivo was followed by vascularization, osteoclastic resorption of the cartilage template, and appearance of hematopoietic foci. This study reveals the capacity of human MSC to generate bone tissue via an endochondral program and provides a valid model to study mechanisms governing bone development. Most importantly, this process could generate advanced grafts for bone regeneration by invoking a "developmental engineering" paradigm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
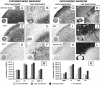


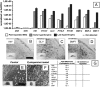
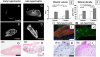
References
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. - PubMed
-
- Noonan KJ, Hunziker EB, Nessler J, Buckwalter JA. Changes in cell, matrix compartment, and fibrillar collagen volumes between growth-plate zones. J Orthop Res. 1998;16:500–508. - PubMed
-
- Bianco P, Cancedda FD, Riminucci M, Cancedda R. Bone formation via cartilage models: The “borderline” chondrocyte. Matrix Biol. 1998;17:185–192. - PubMed
-
- Ashton BA, et al. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

