4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines
- PMID: 20406989
- PMCID: PMC2872136
- DOI: 10.1158/0008-5472.CAN-09-4480
4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines
Abstract
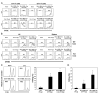
Therapeutic subunit vaccines based on tumor-associated antigens (TAA) represent an attractive approach for the treatment of cancer. However, poor immunogenicity of TAAs requires potent adjuvants for therapeutic efficacy. We recently proposed the tumor necrosis factor family costimulatory ligands as potential adjuvants for therapeutic vaccines and, hence, generated a soluble form of 4-1BBL chimeric with streptavidin (SA-4-1BBL) that has pleiotropic effects on cells of innate, adaptive, and regulatory immunity. We herein tested whether these effects can translate into effective cancer immunotherapy when SA-4-1BBL was also used as a vehicle to deliver TAAs in vivo to dendritic cells (DCs) constitutively expressing the 4-1BB receptor. SA-4-1BBL was internalized by DCs upon receptor binding and immunization with biotinylated antigens conjugated to SA-4-1BBL resulted in increased antigen uptake and cross-presentation by DCs, leading to the generation of effective T-cell immune responses. Conjugate vaccines containing human papillomavirus 16 E7 oncoprotein or survivin as a self-TAA had potent therapeutic efficacy against TC-1 cervical and 3LL lung carcinoma tumors, respectively. Therapeutic efficacy of the vaccines was associated with increased CD4(+) T and CD8(+) T-cell effector and memory responses and higher intratumoral CD8(+) T effector/CD4(+)CD25(+)Foxp3(+) T regulatory cell ratio. Thus, potent pleiotropic immune functions of SA-4-1BBL combined with its ability to serve as a vehicle to increase the delivery of antigens to DCs in vivo endow this molecule with the potential to serve as an effective immunomodulatory component of therapeutic vaccines against cancer and chronic infections.
(c)2010 AACR.
Conflict of interest statement
Figures

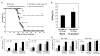


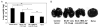
References
-
- Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. - PubMed
-
- Ishii KJ, Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol. 2007;27:363–71. - PubMed
-
- Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

