Role of N-linked glycosylation in biosynthesis, trafficking, and function of the human glucagon-like peptide 1 receptor
- PMID: 20407008
- PMCID: PMC2904048
- DOI: 10.1152/ajpendo.00067.2010
Role of N-linked glycosylation in biosynthesis, trafficking, and function of the human glucagon-like peptide 1 receptor
Abstract
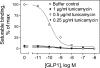
The family B G protein-coupled glucagon-like peptide 1 (GLP-1) receptor is an important drug target for treatment of type 2 diabetes. Like other family members, the GLP-1 receptor is a glycosylated membrane protein that contains three potential sites for N-linked glycosylation within the functionally important extracellular amino-terminal domain. However, the roles for each potential site of glycosylation in receptor biosynthesis, trafficking, and function are not known. In this work, we demonstrated that tunicamycin inhibition of glycosylation of the GLP-1 receptor expressed in CHO cells interfered with biosynthesis and intracellular trafficking, thereby eliminating natural ligand binding. To further investigate the roles of each of the glycosylation sites, site-directed mutagenesis was performed to eliminate these sites individually and in aggregate. Our results showed that mutation of each of the glycosylation sites individually did not interfere with receptor expression on the cell surface, ligand binding, and biological activity. However, simultaneous mutation of two or three glycosylation sites resulted in almost complete loss of GLP-1 binding and severely impaired biological activity. Immunostaining studies demonstrated receptor biosynthesis but aberrant trafficking, with most of the receptor trapped in the endoplasmic reticulum and golgi compartments and little of the receptor expressed on the cell surface. Interestingly, surface expression, ligand binding, and biological activity of these mutants improved significantly when biosynthesis was slowed using low temperature (30 degrees C). These data suggest that N-linked glycosylation of the GLP-1 receptor is important for its normal folding and trafficking to the cell surface.
Figures
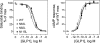
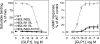

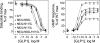
References
-
- Anukanth A, Khorana HG. Structure and function in rhodopsin. Requirements of a specific structure for the intradiscal domain. J Biol Chem 269: 19738–19744, 1994 - PubMed
-
- Assil IQ, Abou-Samra AB. N-glycosylation of CRF receptor type 1 is important for its ligand-specific interaction. Am J Physiol Endocrinol Metab 281: E1015–E1021, 2001 - PubMed
-
- Bisello A, Greenberg Z, Behar V, Rosenblatt M, Suva LJ, Chorev M. Role of glycosylation in expression and function of the human parathyroid hormone/parathyroid hormone-related protein receptor. Biochemistry 35: 15890–15895, 1996 - PubMed
-
- Breitfeld PP, Rup D, Schwartz AL. Influence of the N-linked oligosaccharides on the biosynthesis, intracellular routing, and function of the human asialoglycoprotein receptor. J Biol Chem 259: 10414–10421, 1984 - PubMed
-
- Buhlmann N, Aldecoa A, Leuthauser K, Gujer R, Muff R, Fischer JA, Born W. Glycosylation of the calcitonin receptor-like receptor at Asn(60) or Asn(112) is important for cell surface expression. FEBS Lett 486: 320–324, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

