Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells
- PMID: 20407012
- PMCID: PMC2872686
- DOI: 10.1158/1541-7786.MCR-09-0493
Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells
Abstract
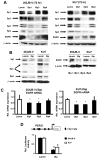
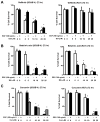
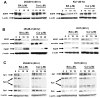
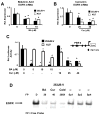
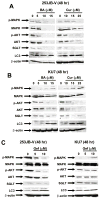
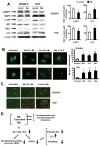
The epidermal growth factor receptor (EGFR) is an important chemotherapeutic target for tyrosine kinase inhibitors and antibodies that block the extracellular domain of EGFR. Betulinic acid (BA) and curcumin inhibited bladder cancer cell growth and downregulated specificity protein (Sp) transcription factors, and this was accompanied by decreased expression of EGFR mRNA and protein levels. EGFR, a putative Sp-regulated gene, was also decreased in cells transfected with a cocktail (iSp) containing small inhibitory RNAs for Sp1, Sp3, and Sp4, and RNA interference with individual Sp knockdown indicated that EGFR expression was primarily regulated by Sp1 and Sp3. BA, curcumin, and iSp also decreased phosphorylation of Akt in these cells, and downregulation of EGFR by BA, curcumin, and iSp was accompanied by induction of LC3 and autophagy, which is consistent with recent studies showing that EGFR suppresses autophagic cell death. The results show that EGFR is an Sp-regulated gene in bladder cancer, and drugs such as BA and curcumin that repress Sp proteins also ablate EGFR expression. Thus, compounds such as curcumin and BA that downregulate Sp transcription factors represent a novel class of anticancer drugs that target EGFR in bladder cancer cells and tumors by inhibiting receptor expression.
(c)2010 AACR.
Figures
Similar articles
-
Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs.BMC Cancer. 2012 Nov 30;12:564. doi: 10.1186/1471-2407-12-564. BMC Cancer. 2012. PMID: 23194063 Free PMC article.
-
Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors.BMC Cancer. 2011 Aug 24;11:371. doi: 10.1186/1471-2407-11-371. BMC Cancer. 2011. PMID: 21864401 Free PMC article.
-
Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: role of Sp transcription factors and microRNA-27a:ZBTB10.Mol Carcinog. 2013 Aug;52(8):591-602. doi: 10.1002/mc.21893. Epub 2012 Mar 7. Mol Carcinog. 2013. PMID: 22407812 Free PMC article.
-
Specificity Proteins (Sp) and Cancer.Int J Mol Sci. 2023 Mar 8;24(6):5164. doi: 10.3390/ijms24065164. Int J Mol Sci. 2023. PMID: 36982239 Free PMC article. Review.
-
Specificity Protein Transcription Factors and Cancer: Opportunities for Drug Development.Cancer Prev Res (Phila). 2018 Jul;11(7):371-382. doi: 10.1158/1940-6207.CAPR-17-0407. Epub 2018 Mar 15. Cancer Prev Res (Phila). 2018. PMID: 29545399 Review.
Cited by
-
Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs.BMC Cancer. 2012 Nov 30;12:564. doi: 10.1186/1471-2407-12-564. BMC Cancer. 2012. PMID: 23194063 Free PMC article.
-
Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation.J Biol Chem. 2010 Aug 13;285(33):25332-44. doi: 10.1074/jbc.M109.095240. Epub 2010 Jun 9. J Biol Chem. 2010. PMID: 20538607 Free PMC article.
-
The Histone Methyltransferase Gene G9A Is Regulated by Nuclear Receptor 4A1 in Alveolar Rhabdomyosarcoma Cells.Mol Cancer Ther. 2021 Mar;20(3):612-622. doi: 10.1158/1535-7163.MCT-20-0474. Epub 2020 Dec 4. Mol Cancer Ther. 2021. PMID: 33277444 Free PMC article.
-
Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death.J Exp Clin Cancer Res. 2019 Jun 13;38(1):254. doi: 10.1186/s13046-019-1234-8. J Exp Clin Cancer Res. 2019. PMID: 31196210 Free PMC article.
-
Bisdemethoxycurcumin Enhances the Sensitivity of Non-small Cell Lung Cancer Cells to Icotinib via Dual Induction of Autophagy and Apoptosis.Int J Biol Sci. 2020 Mar 5;16(9):1536-1550. doi: 10.7150/ijbs.40042. eCollection 2020. Int J Biol Sci. 2020. PMID: 32226300 Free PMC article.
References
-
- Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. - PubMed
-
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and Krüppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–60. - PubMed
-
- Ammendola R, Mesuraca M, Russo T, Cimino F. Sp1 DNA binding efficiency is highly reduced in nuclear extracts from aged rat tissues. J Biol Chem. 1992;267:17944–8. - PubMed
-
- Adrian GS, Seto E, Fischbach KS, et al. YY1 and Sp1 transcription factors bind the human transferrin gene in an age-related manner. J Gerontol A Biol Sci Med Sci. 1996;51:B66–B75. - PubMed
-
- Oh JE, Han JA, Hwang ES. Downregulation of transcription factor, Sp1, during cellular senescence. Biochem Biophys Res Commun. 2007;353:86–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

