Arabidopsis lyrata small RNAs: transient MIRNA and small interfering RNA loci within the Arabidopsis genus
- PMID: 20407023
- PMCID: PMC2879747
- DOI: 10.1105/tpc.110.073882
Arabidopsis lyrata small RNAs: transient MIRNA and small interfering RNA loci within the Arabidopsis genus
Abstract
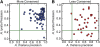
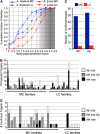
Twenty-one-nucleotide microRNAs (miRNAs) and 24-nucleotide Pol IV-dependent small interfering RNAs (p4-siRNAs) are the most abundant types of small RNAs in angiosperms. Some miRNAs are well conserved among different plant lineages, whereas others are less conserved, and it is not clear whether less-conserved miRNAs have the same functionality as the well conserved ones. p4-siRNAs are broadly produced in the Arabidopsis genome, sometimes from active hot spot loci, but it is unknown whether individual p4-siRNA hot spots are retained as hot spots between plant species. In this study, we compare small RNAs in two closely related species (Arabidopsis thaliana and Arabidopsis lyrata) and find that less-conserved miRNAs have high rates of divergence in MIRNA hairpin structures, mature miRNA sequences, and target-complementary sites in the other species. The fidelity of miRNA biogenesis from many less-conserved MIRNA hairpins frequently deteriorates in the sister species relative to the species of first discovery. We also observe that p4-siRNA occupied loci have a slight tendency to be retained as p4-siRNA loci between species, but the most active A. lyrata p4-siRNA hot spots are generally not syntenic to the most active p4-siRNA hot spots of A. thaliana. Altogether, our findings indicate that many MIRNAs and most p4-siRNA hot spots are rapidly changing and evolutionarily transient within the Arabidopsis genus.
Figures




Comment in
-
MicroRNA evolution in the genus Arabidopsis.Plant Cell. 2010 Apr;22(4):994. doi: 10.1105/tpc.110.220411. Epub 2010 Apr 20. Plant Cell. 2010. PMID: 20407026 Free PMC article. No abstract available.
Similar articles
-
MicroRNA gene evolution in Arabidopsis lyrata and Arabidopsis thaliana.Plant Cell. 2010 Apr;22(4):1074-89. doi: 10.1105/tpc.110.073999. Epub 2010 Apr 20. Plant Cell. 2010. PMID: 20407027 Free PMC article.
-
Rapid divergence and high diversity of miRNAs and miRNA targets in the Camelineae.Plant J. 2015 Feb;81(4):597-610. doi: 10.1111/tpj.12754. Epub 2015 Jan 21. Plant J. 2015. PMID: 25557441
-
Small RNAs serve as a genetic buffer against genomic shock in Arabidopsis interspecific hybrids and allopolyploids.Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17835-40. doi: 10.1073/pnas.0907003106. Epub 2009 Oct 5. Proc Natl Acad Sci U S A. 2009. PMID: 19805056 Free PMC article.
-
Revisiting Criteria for Plant MicroRNA Annotation in the Era of Big Data.Plant Cell. 2018 Feb;30(2):272-284. doi: 10.1105/tpc.17.00851. Epub 2018 Jan 17. Plant Cell. 2018. PMID: 29343505 Free PMC article. Review.
-
Plant Small RNAs: Their Biogenesis, Regulatory Roles, and Functions.Annu Rev Plant Biol. 2023 May 22;74:21-51. doi: 10.1146/annurev-arplant-070122-035226. Epub 2023 Feb 28. Annu Rev Plant Biol. 2023. PMID: 36854480 Review.
Cited by
-
MicroRNA evolution in the genus Arabidopsis.Plant Cell. 2010 Apr;22(4):994. doi: 10.1105/tpc.110.220411. Epub 2010 Apr 20. Plant Cell. 2010. PMID: 20407026 Free PMC article. No abstract available.
-
Characterization and expression profiling of microRNAs in response to plant feeding in two host-plant strains of the lepidopteran pest Spodoptera frugiperda.BMC Genomics. 2018 Nov 6;19(1):804. doi: 10.1186/s12864-018-5119-6. BMC Genomics. 2018. PMID: 30400811 Free PMC article.
-
Imprinted green beards: a little less than kin and more than kind.Biol Lett. 2013 Oct 16;9(6):20130199. doi: 10.1098/rsbl.2013.0199. Print 2013. Biol Lett. 2013. PMID: 24132086 Free PMC article.
-
Identification of novel and conserved miRNAs involved in pollen development in Brassica campestris ssp. chinensis by high-throughput sequencing and degradome analysis.BMC Genomics. 2014 Feb 21;15:146. doi: 10.1186/1471-2164-15-146. BMC Genomics. 2014. PMID: 24559317 Free PMC article.
-
Identification and comparative analysis of cadmium tolerance-associated miRNAs and their targets in two soybean genotypes.PLoS One. 2013 Dec 10;8(12):e81471. doi: 10.1371/journal.pone.0081471. eCollection 2013. PLoS One. 2013. PMID: 24363811 Free PMC article.
References
-
- Allen E., Xie Z., Gustafson A.M., Carrington J.C. (2005). microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

