Netrin-4 induces lymphangiogenesis in vivo
- PMID: 20407033
- PMCID: PMC2902137
- DOI: 10.1182/blood-2009-11-252338
Netrin-4 induces lymphangiogenesis in vivo
Abstract
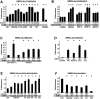
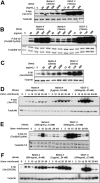
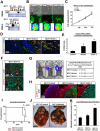
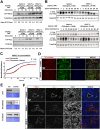
Netrin-4, a laminin-related secreted protein is an axon guidance cue recently shown essential outside of the nervous system, regulating mammary and lung morphogenesis as well as blood vascular development. Here, we show that Netrin-4, at physiologic doses, induces proliferation, migration, adhesion, tube formation and survival of human lymphatic endothelial cells in vitro comparable to well-characterized lymphangiogenic factors fibroblast growth factor-2 (FGF-2), hepatocyte growth factor (HGF), vascular endothelial growth factor-A (VEGF-A), and vascular endothelial growth factor-C (VEGF-C). Netrin-4 stimulates phosphorylation of intracellular signaling components Akt, Erk and S6, and their specific inhibition antagonizes Netrin-4-induced proliferation. Although Netrin receptors Unc5B and neogenin, are expressed by human lymphatic endothelial cells, suppression of either or both does not suppress Netrin-4-promoted in vitro effects. In vivo, Netrin-4 induces growth of lymphatic and blood vessels in the skin of transgenic mice and in breast tumors. Its overexpression in human and mouse mammary carcinoma cancer cells leads to enhanced metastasis. Finally, Netrin-4 stimulates in vitro and in vivo lymphatic permeability by activating small GTPases and Src family kinases/FAK, and down-regulating tight junction proteins. Together, these data provide evidence that Netrin-4 is a lymphangiogenic factor contributing to tumor dissemination and represents a potential target to inhibit metastasis formation.
Figures

References
-
- Sleeman JP, Thiele W. Tumor metastasis and the lymphatic vasculature. Int J Cancer. 2009 Jun 30; - PubMed
-
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49(3):507–521. - PubMed
-
- Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8(4):296–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

