Iron control of erythroid development by a novel aconitase-associated regulatory pathway
- PMID: 20407036
- PMCID: PMC2904585
- DOI: 10.1182/blood-2009-10-251496
Iron control of erythroid development by a novel aconitase-associated regulatory pathway
Abstract
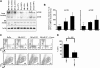
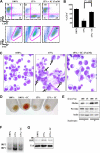
Human red cell differentiation requires the action of erythropoietin on committed progenitor cells. In iron deficiency, committed erythroid progenitors lose responsiveness to erythropoietin, resulting in hypoplastic anemia. To address the basis for iron regulation of erythropoiesis, we established primary hematopoietic cultures with transferrin saturation levels that restricted erythropoiesis but permitted granulopoiesis and megakaryopoiesis. Experiments in this system identified as a critical regulatory element the aconitases, multifunctional iron-sulfur cluster proteins that metabolize citrate to isocitrate. Iron restriction suppressed mitochondrial and cytosolic aconitase activity in erythroid but not granulocytic or megakaryocytic progenitors. An active site aconitase inhibitor, fluorocitrate, blocked erythroid differentiation in a manner similar to iron deprivation. Exogenous isocitrate abrogated the erythroid iron restriction response in vitro and reversed anemia progression in iron-deprived mice. The mechanism for aconitase regulation of erythropoiesis most probably involves both production of metabolic intermediates and modulation of erythropoietin signaling. One relevant signaling pathway appeared to involve protein kinase Calpha/beta, or possibly protein kinase Cdelta, whose activities were regulated by iron, isocitrate, and erythropoietin.
Figures





References
-
- Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96(3):823–833. - PubMed
-
- Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105–131. - PubMed
-
- Shaw GC, Cope JJ, Li L, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440(7080):96–100. - PubMed
-
- Kimura H, Finch CA, Adamson JW. Hematopoiesis in the rat: quantitation of hematopoietic progenitors and the response to iron deficiency anemia. J Cell Physiol. 1986;126(2):298–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

