A review of the gastrointestinal safety data--a gastroenterologist's perspective
- PMID: 20407138
- PMCID: PMC2857792
- DOI: 10.1093/rheumatology/keq058
A review of the gastrointestinal safety data--a gastroenterologist's perspective
Abstract
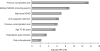
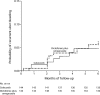
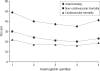
Although NSAIDs have a well-established place for certain indications in the management of OA and RA, they are associated with significant gastrointestinal (GI) toxicity. The risk of NSAID-related upper GI events, such as dyspepsia or peptic ulcer and complications such as perforation or bleeding, is well characterized. Non-selective NSAIDs increase the risk of peptic ulcer disease approximately 5-fold, and that of upper GI bleeding 4-fold, whereas selective cyclo-oxygenase-2 (COX) inhibitors are associated with a significantly lower GI toxicity than non-selective agents. There is evidence that, while the incidence of NSAID-related upper GI complications has decreased in recent years, that of lower GI complications is increasing. Observational studies and analyses from studies, primarily designed to investigate upper GI events, suggest that lower GI complications are relatively common in NSAID users and that COX-2 selective inhibitors are associated with a lower risk of these events. Such events have been poorly characterized, but are associated with significant mortality; indeed, they may have even more serious consequences than the better characterized upper GI events. There is thus a strong case for evaluating the impact of such complications in prospective outcome studies. To facilitate such studies a new endpoint, Clinically Significant Upper or Lower GI Events, has been introduced that captures both upper and lower GI events.
Figures

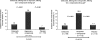
References
-
- Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ for the VACT Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee. JAMA. 2002;287:64–71. - PubMed
-
- Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–55. - PMC - PubMed
-
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–15. - PubMed
-
- Paulus HE. FDA Arthritis Advisory Committee meeting: serious gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs; drug-containing renal and biliary stones; diclofenac and carprofen approved. Arthritis Rheum. 1988;31:1450–1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

