Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents
- PMID: 20407211
- PMCID: PMC2860903
- DOI: 10.1172/JCI41356
Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents
Abstract
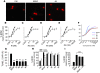
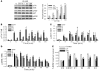
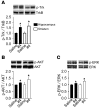
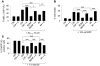
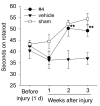
Brain-derived neurotrophic factor (BDNF) activates the receptor tropomyosin-related kinase B (TrkB) with high potency and specificity, promoting neuronal survival, differentiation, and synaptic function. Correlations between altered BDNF expression and/or function and mechanism(s) underlying numerous neurodegenerative conditions, including Alzheimer disease and traumatic brain injury, suggest that TrkB agonists might have therapeutic potential. Using in silico screening with a BDNF loop-domain pharmacophore, followed by low-throughput in vitro screening in mouse fetal hippocampal neurons, we have efficiently identified small molecules with nanomolar neurotrophic activity specific to TrkB versus other Trk family members. Neurotrophic activity was dependent on TrkB and its downstream targets, although compound-induced signaling activation kinetics differed from those triggered by BDNF. A selected prototype compound demonstrated binding specificity to the extracellular domain of TrkB. In in vitro models of neurodegenerative disease, it prevented neuronal degeneration with efficacy equal to that of BDNF, and when administered in vivo, it caused hippocampal and striatal TrkB activation in mice and improved motor learning after traumatic brain injury in rats. These studies demonstrate the utility of loop modeling in drug discovery and reveal what we believe to be the first reported small molecules derived from a targeted BDNF domain that specifically activate TrkB.We propose that these compounds constitute a novel group of tools for the study of TrkB signaling and may provide leads for developing new therapeutic agents for neurodegenerative diseases.
Figures
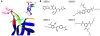
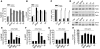
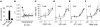
Comment in
-
Neurodegenerative disease: BDNF copycats.Nat Rev Drug Discov. 2010 Jun;9(6):433. doi: 10.1038/nrd3190. Nat Rev Drug Discov. 2010. PMID: 20514068 No abstract available.
Similar articles
-
A small molecule TrkB/TrkC neurotrophin receptor co-activator with distinctive effects on neuronal survival and process outgrowth.Neuropharmacology. 2016 Nov;110(Pt A):343-361. doi: 10.1016/j.neuropharm.2016.06.015. Epub 2016 Jun 19. Neuropharmacology. 2016. PMID: 27334657
-
VGF and Its C-Terminal Peptide TLQP-62 Regulate Memory Formation in Hippocampus via a BDNF-TrkB-Dependent Mechanism.J Neurosci. 2015 Jul 15;35(28):10343-56. doi: 10.1523/JNEUROSCI.0584-15.2015. J Neurosci. 2015. PMID: 26180209 Free PMC article.
-
TrkB/BDNF signaling pathway and its small molecular agonists in CNS injury.Life Sci. 2024 Jan 1;336:122282. doi: 10.1016/j.lfs.2023.122282. Epub 2023 Nov 24. Life Sci. 2024. PMID: 38008209 Review.
-
Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb(2+): implications for an environmental basis of neurodevelopmental disorders.Toxicol Sci. 2012 May;127(1):277-95. doi: 10.1093/toxsci/kfs090. Epub 2012 Feb 17. Toxicol Sci. 2012. PMID: 22345308 Free PMC article.
-
The Role of Brain-Derived Neurotrophic Factor as an Essential Mediator in Neuronal Functions and the Therapeutic Potential of Its Mimetics for Neuroprotection in Neurologic and Psychiatric Disorders.Molecules. 2025 Feb 12;30(4):848. doi: 10.3390/molecules30040848. Molecules. 2025. PMID: 40005159 Free PMC article. Review.
Cited by
-
The Neurotrophic-Like Effect of Carvacrol: Perspective for Axonal and Synaptic Regeneration.Neurotox Res. 2021 Jun;39(3):886-896. doi: 10.1007/s12640-021-00341-1. Epub 2021 Mar 5. Neurotox Res. 2021. PMID: 33666886
-
Smith-Magenis syndrome protein RAI1 regulates body weight homeostasis through hypothalamic BDNF-producing neurons and neurotrophin downstream signalling.Elife. 2023 Nov 13;12:RP90333. doi: 10.7554/eLife.90333. Elife. 2023. PMID: 37956053 Free PMC article.
-
Beyond traditional pharmacology: new tools and approaches.Br J Pharmacol. 2015 Jul;172(13):3229-41. doi: 10.1111/bph.13066. Epub 2015 Jun 10. Br J Pharmacol. 2015. PMID: 25572005 Free PMC article. Review.
-
Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer's Disease.Sci Rep. 2016 Jun 6;6:27358. doi: 10.1038/srep27358. Sci Rep. 2016. PMID: 27264956 Free PMC article.
-
Low molecular weight, non-peptidic agonists of TrkA receptor with NGF-mimetic activity.Cell Death Dis. 2012 Jul 5;3(7):e339. doi: 10.1038/cddis.2012.80. Cell Death Dis. 2012. PMID: 22764098 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

