Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning
- PMID: 20407224
- PMCID: PMC2968769
- DOI: 10.1159/000313399
Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning
Abstract
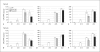
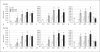
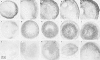
Induction of chondrogenesis in mesenchymal stem cells (MSCs) with TGF-beta leads to a hypertrophic phenotype. The hypertrophic maturation of the chondrocytes is dependent on the timed removal of TGF-beta and sensitive to hypertrophy-promoting agents in vitro. In this study, we have investigated whether TGF-beta3, which has been shown to be more prochondrogenic compared to TGF-beta1, similarly enhances terminal differentiation in an in vitro hypertrophy model of chondrogenically differentiating MSCs. In addition, we tested the impact of the time of chondrogenic conditioning on the enhancement of hypertrophy. MSCs were chondrogenically differentiated in pellet culture in medium containing TGF-beta1 or TGF-beta3. After 2 or 4 weeks, chondrogenic medium was switched to hypertrophy-inducing medium for 2 weeks. Aggregates were analyzed histologically and biochemically on days 14, 28 and 42. The switch to hypertrophy medium after 14 days induced hypertrophic cell morphology and significant increase in alkaline phosphatase activity compared to the chondrogenesis only control using both TGF-beta1 and TGF-beta3. After 28 days predifferentiation, differences between hypertrophic and control groups diminished compared to 14 days predifferentiation. In conclusion, chondrogenic conditioning with both TGF-beta isoforms similarly induced hypertrophy in our experiment and allowed the enhancement of the hypertrophic chondrocyte phenotype by hypertrophic medium. Enhancement of hypertrophy was seen more clearly after the shorter chondrogenic conditioning. Therefore, to utilize this experimental model as a tool to study hypertrophy in MSC chondrogenesis, a predifferentiation period of 14 days is recommended.
Copyright 2010 S. Karger AG, Basel.
Figures

Similar articles
-
Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis.Arthritis Rheum. 2010 Sep;62(9):2696-706. doi: 10.1002/art.27565. Arthritis Rheum. 2010. PMID: 20496422
-
Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis.Int Orthop. 2013 May;37(5):945-51. doi: 10.1007/s00264-013-1800-1. Epub 2013 Jan 31. Int Orthop. 2013. PMID: 23371427 Free PMC article.
-
Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis.Biotechnol Bioeng. 2006 Apr 20;93(6):1152-63. doi: 10.1002/bit.20828. Biotechnol Bioeng. 2006. PMID: 16470881
-
PTHrP isoforms have differing effect on chondrogenic differentiation and hypertrophy of mesenchymal stem cells.Biochem Biophys Res Commun. 2012 May 18;421(4):819-24. doi: 10.1016/j.bbrc.2012.04.096. Epub 2012 Apr 25. Biochem Biophys Res Commun. 2012. PMID: 22554518
-
In Vitro Induction of Hypertrophic Chondrocyte Differentiation of Naïve MSCs by Strain.Cells. 2024 Dec 30;14(1):25. doi: 10.3390/cells14010025. Cells. 2024. PMID: 39791725 Free PMC article.
Cited by
-
HydraPsiSeq: a method for systematic and quantitative mapping of pseudouridines in RNA.Nucleic Acids Res. 2020 Nov 4;48(19):e110. doi: 10.1093/nar/gkaa769. Nucleic Acids Res. 2020. PMID: 32976574 Free PMC article.
-
Topographically and Chemically Enhanced Textile Polycaprolactone Scaffolds for Tendon and Ligament Tissue Engineering.Polymers (Basel). 2024 Feb 9;16(4):488. doi: 10.3390/polym16040488. Polymers (Basel). 2024. PMID: 38399866 Free PMC article.
-
Combination of kartogenin and transforming growth factor-β3 supports synovial fluid-derived mesenchymal stem cell-based cartilage regeneration.Am J Transl Res. 2019 Apr 15;11(4):2056-2069. eCollection 2019. Am J Transl Res. 2019. PMID: 31105817 Free PMC article.
-
Boosting tendon repair: interplay of cells, growth factors and scaffold-free and gel-based carriers.J Exp Orthop. 2018 Jan 5;5(1):1. doi: 10.1186/s40634-017-0117-1. J Exp Orthop. 2018. PMID: 29330711 Free PMC article. Review.
-
Bioimaging: An Useful Tool to Monitor Differentiation of Human Embryonic Stem Cells into Chondrocytes.Ann Biomed Eng. 2016 May;44(5):1845-59. doi: 10.1007/s10439-015-1443-z. Epub 2015 Sep 9. Ann Biomed Eng. 2016. PMID: 26354117 Free PMC article.
References
-
- Bahrami S., Plate U., Dreier R., DuChesne A., Willital G.H., Bruckner P. Endochondral ossification of costal cartilage is arrested after chondrocytes have reached hypertrophic stage of late differentiation. Matrix Biol. 2001;19:707–715. - PubMed
-
- Ballock R.T., Heydemann A., Wakefield L.M., Flanders K.C., Roberts A.B., Sporn M.B. TGF-β1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414–429. - PubMed
-
- Ballock R.T., O'Keefe R.J. Physiology and pathophysiology of the growth plate. Birth Defects Res C Embryo Today. 2003;69:123–143. - PubMed
-
- Barry F., Boynton R.E., Liu B., Murphy J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. - PubMed
-
- Cheung J.O., Hillarby M.C., Ayad S., Hoyland J.A., Jones C.J., Denton J., Thomas J.T., Wallis G.A., Grant M.E. A novel cell culture model of chondrocyte differentiation during mammalian endochondral ossification. J Bone Miner Res. 2001;16:309–318. - PubMed

