High-frequency alloreactive T cells augment effector function of low-frequency CD8+ T-cell responses under CD28/CD154 blockade
- PMID: 20407401
- PMCID: PMC2935314
- DOI: 10.1097/TP.0b013e3181df53dc
High-frequency alloreactive T cells augment effector function of low-frequency CD8+ T-cell responses under CD28/CD154 blockade
Abstract
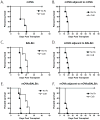
Background: Blockade of costimulatory molecules is a potent method of inducing long-term graft survival. We have previously addressed the issue of donor-reactive T-cell precursor frequency on relative costimulation dependence and found that the presence of a high precursor frequency of donor-reactive CD8 T cells resulted in costimulation blockade-resistant graft rejection, whereas the presence of a low-frequency donor-reactive population did not. To address the mechanisms by which high-frequency T cells obviated the requirement for costimulation, we asked whether a low-frequency population responding concomitantly with a high-frequency response also demonstrated costimulation independence.
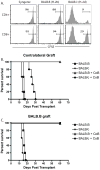
Methods: A model system was established in which B6 mice containing a low frequency of anti-membrane bound chicken ovalbumin (mOVA) responders and a high frequency of anti-BALB/c responders received a skin graft from B6.mOVAxBALB/c F1 donors in the presence or absence of cytotoxic T-lymphocyte antigen-4 Ig/anti-CD154 costimulatory blockade.
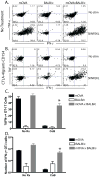
Results: The results revealed that in the presence of costimulation blockade, high-frequency anti-BALB/c T cells augmented the effector activity of low-frequency anti-mOVA T cells, but it did not enhance the accumulation of anti-mOVA T cells capable of mediating graft rejection.
Conclusions: These results demonstrate that both antigen-specific and antigen-independent factors contribute to the relative costimulation independence of high-frequency T-cell responses.
Figures

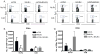
References
-
- Wirth T, Harty JT. Initial TCR transgenic precursor frequency alters functional behaviour of CD8 T cells responding to acute infection. Adv Exp Med Biol. 2009;633:71. - PubMed
-
- Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312 (5770):114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

